RevBio Receives Both FDA Approval to Expand its Clinical Trial and CMS Reimbursement for its Regenerative Bone Adhesive for Cranial Flap Fixation
RevBio Receives Both FDA Approval to Expand its Clinical Trial and CMS Reimbursement for its Regenerative Bone Adhesive for Cranial Flap Fixation
The Expansion of this Clinical Trial will Broaden the Clinical Use Cases for TETRANITE® and Establish Reimbursement Coverage
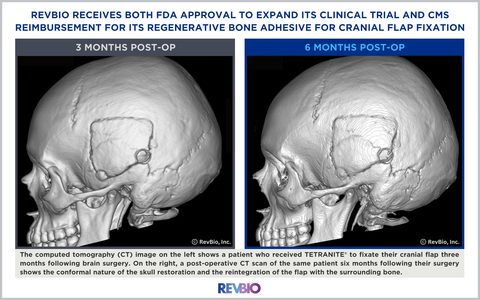
The computed tomography (CT) image on the left shows a patient who received TETRANITE® to fixate their cranial flap three months following brain surgery. On the right, a post-operative CT scan of the same patient six months following their surgery shows the conformal nature of the skull restoration and the reintegration of the flap with the surrounding bone. (Photo: Business Wire)
LOWELL, Mass.--(BUSINESS WIRE)--RevBio, Inc., announced that it has received FDA approval to expand its ongoing clinical trial to immediately fixate cranial flaps using TETRANITE®, the company’s bone adhesive biomaterial. The company also received reimbursement coverage from the Centers for Medicare and Medicaid (“CMS”) for the use of TETRANITE to replace metal plates and screws.
Previously, RevBio received FDA approval to initiate a first-in-human clinical study for an initial five patients to restore cranial flaps following craniotomy procedures and to repair extradural use cases where intentional durotomies are not required. The approval of this clinical trial expansion is a result of the successful demonstration of safety in the first five patients treated with TETRANITE. The neurosurgeon investigators involved in this study will now be able to use TETRANITE in intradural use cases, such as tumor resection surgeries and other cranial procedures, which require intentional durotomies.
“Metal plates and screws can produce radiographic artifacts which make it difficult to accurately interpret patient imaging following surgery. Furthermore, traditional cranial flap closure methods do not lead to the full osseous reintegration of the flap with the skull, resulting in issues with flap movement, patient pain, and the potential for post-surgical infection. Because TETRANITE eliminates radiographic artifacts and results in osseous union of bone flaps with the surrounding skull, it could become the new standard of care for cranial surgeries,” said Kevin T. Foley, MD, professor in the Departments of Neurosurgery and Orthopedic Surgery & Biomedical Engineering at the University of Tennessee Health Science Center in Memphis, Tennessee, Chairman of Semmes-Murphey Clinic, and Chairman of the Board and Medical Director for the Medical Education & Research Institute (MERI). Dr. Foley also serves as a member of the Board of Directors and Chief Medical Officer for RevBio.
Furthermore, in conjunction with the clinical trial expansion, the Centers for Medicare and Medicaid approved TETRANITE for medical insurance reimbursement when used to replace metal plates and screws for cranial flap fixation. Private insurance companies will also typically provide reimbursement for CMS approved products. As a result, RevBio can now charge hospitals for this product.
“We are pleased to receive reimbursement approval from CMS during our IDE clinical trial,” said Grayson Allen, CFO/COO of RevBio. “This will enable RevBio to establish a price with healthcare providers, which is a very important step in the overall product commercialization process.”
About RevBio, Inc.
RevBio, Inc., is a clinical stage medical device company developing and commercializing TETRANITE®, a patented, synthetic, injectable, self-setting, and osteoconductive adhesive biomaterial. This novel technology will be indicated for use in dental, cranial, and broader orthopaedic applications as well as animal health. TETRANITE is not yet approved for commercial use.
Contacts
Michael Tiedemann
mtiedemann@revbio.com