Novotech Releases Comprehensive Report on the Global Clinical Trial Landscape for Small Cell Lung Cancer (SCLC)
Novotech Releases Comprehensive Report on the Global Clinical Trial Landscape for Small Cell Lung Cancer (SCLC)
SAN FRANCISCO--(BUSINESS WIRE)--Novotech, the global full-service clinical Contract Research Organization (CRO), has published a new report, Small Cell Lung Cancer: Global Clinical Trial Landscape 2024, providing an in-depth analysis of current trends, challenges, and opportunities in SCLC clinical trials worldwide.
Novotech, the global full-service clinical Contract Research Organization (CRO), has published a new report, Small Cell Lung Cancer: Global Clinical Trial Landscape 2024.
Share
This report provides insights into oncology, highlighting Novotech’s commitment to advancing therapeutic research.
Key Insights from the Report:
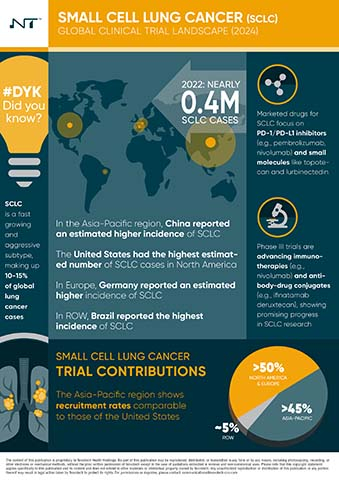
- Epidemiology and Incidence Patterns – Analysis of global SCLC distribution, with Asia showing the highest incidence (63%), followed by Europe and North America. These insights are critical for crafting region-specific trials and recruitment strategies.
- Drug Development Pipeline – Overview of the SCLC pipeline, emphasizing innovative treatments like immune checkpoint inhibitors and targeted therapies. This section highlights the pipeline's multi-stage growth and the expanding role of immunotherapies in SCLC treatment.
- Funding and Investment Landscape – Examination of public and private funding trends, noting increased venture capital investment in SCLC research, primarily from China and the U.S., reflecting strong market potential for new treatments.
- Evolving Therapeutic Strategies – Review of advanced treatment options, including radiotherapy, gene therapies, and personalized approaches, highlighting the role of precision medicine in enhancing SCLC patient outcomes.
- Operational Challenges and Solutions – Exploration of the complexities in SCLC trials, such as patient recruitment and regulatory compliance, particularly for multi-regional studies.
Download the full report for insights into the future of SCLC trials and strategies to leverage these trends for clinical success.
About Novotech Novotech-CRO.com
Founded in 1997, Novotech is a global full-service clinical Contract Research Organization (CRO) focused on partnering with biotech companies to accelerate the development of advanced and novel therapeutics at every phase.
Recognized for its industry-leading contributions, Novotech has received numerous prestigious awards, including the Frost &Sullivan 2024 Global Biotech CRO of the year award, Clinical Trials Arena Excellence Awards 2024, 2024 Employer of Choice, 2024 Great Place to Work in the US, 2024 Brandon Hall Gold Award, CRO Leadership Award 2023, the Asia Pacific Cell & Gene Therapy Clinical Trials Excellence 2023, the Asia-Pacific Contract Research Organization Company of the Year Award since 2006.
The Company offers a comprehensive suite of services including laboratories, Phase I facilities, drug development consulting, regulatory expertise, and has experience with over 5,000 clinical projects, including Phase I to Phase IV clinical trials and bioequivalence studies. With a presence in 34 office locations and a dedicated team of 3,000+ professionals worldwide, Novotech is a trusted end-to-end strategic partner of choice.
For more information or to speak to an expert team member visit www.Novotech-CRO.com
Contacts
Media Contact
Toyna Chin
mediacontact@novotech-cro.com
USA: +1 415 364 8135