CroíValve Announces $16 Million Equity Financing to Fund US Early Feasibility Study of Novel Transcatheter Tricuspid Device
CroíValve Announces $16 Million Equity Financing to Fund US Early Feasibility Study of Novel Transcatheter Tricuspid Device
DUBLIN--(BUSINESS WIRE)--CroíValve, a pioneering medical device company focused on the development of a novel transcatheter device for the treatment of tricuspid regurgitation, announced today the closing of $16 million Series B financing. The round, led by the MedTech & Irrus Syndicates, included participation from all current investors, Ascentifi, Furthr, Broadview Ventures, Atlantic Bridge University Fund, Enterprise Ireland, Elkstone & SOSV, along with a new investor, IAG Capital Partners. CroíValve welcomes Daniel Gottlieb, a Director with Broadview Ventures, to its Board of Directors. Daniel has 20 years of experience with medical device and biotechnology companies in business development, marketing and strategy and previously served as an Observer on the CroíValve Board. Additionally, as a Venture Partner with IAG Capital Partners, Ehsan Jabbarzadeh will join the CroíValve Board as an observer.
The new financing follows a successful European First in Human study, the TANDEM I study, which demonstrated sustained reductions in tricuspid regurgitation and marked improvements in patients’ quality of life metrics for patients treated with the DUO™ System:
- 89% of patients experienced > 15-point improvement in the Kansas City Cardiomyopathy Questionnaire (KCCQ)
- 100% of patients achieved a New York Heart Association (NYHA) classification of I or II post treatment
- substantial (+63m average) improvement in the 6-minute walk distance (6MWD)
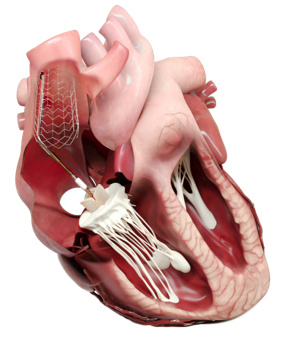
Proceeds from the financing will be used to fund the TANDEM II Early Feasibility Study, an FDA-approved, prospective, multicenter study to evaluate the safety and performance of the DUO™ System in patients with severe or greater symptomatic tricuspid regurgitation (TR). Tricuspid regurgitation is a severe heart condition that occurs when the tricuspid valve in the right side of the heart fails to close properly. TR affects over 1.6 million people in the US and is associated with significant morbidity and reduced life expectancy.
The DUO™ System is a novel transcatheter heart valve that works in tandem with the native tricuspid valve to restore valve function while leaving the patient’s right heart and native valve apparatus untouched through an innovative anchoring mechanism. Designed to treat a broad patient population, the DUO™ System accommodates the heterogeneity of patient anatomies while avoiding contact with critical structures in the right heart which could lead to complications. The DUO™ System is also designed to enable a straightforward procedure and reduce the procedural complexity seen with other devices. The procedure is less reliant on expert intraprocedural imaging, enabling a short learning curve with predictable and stable outcomes.
“I believe the DUO™ system can simplify the treatment of TR patients, with a predictable procedure that can be performed with standard imaging techniques. Additionally, with minimal anatomical exclusions, it can reach a broad population. It has the potential to emerge as a meaningful advancement in the field of TR treatment,” said Martin B. Leon, MD, CroíValve's Clinical Advisory Board Chair and Mallah Family Professor of Cardiology at the Columbia University Irving Medical Center College of Physicians and Surgeons as well as Director of the Columbia Center for Interventional Care (CICC) at New York-Presbyterian Hospital/Columbia University Medical Center.
“This financing is a testament to the unmet need for treating tricuspid regurgitation and the potential of the DUO™ System to treat more patients with a reproducible and intuitive procedure. We are excited to bring this technology to the US, with strong support from our shareholder base who are committed to our mission to transform the lives of patients suffering from tricuspid regurgitation,” said Lucy O’Keeffe, CEO of CroíValve.
About CroíValve
CroíValve is a clinical stage medical device company focused on the development of a novel transcatheter device for the treatment of tricuspid regurgitation with Research, Development and Operations based in Ireland and Clinical and Regulatory based in the United States.
Caution: The DUO™ System is an investigational device and not for sale in any geography.
Contacts
+1 651-270-3084
stella@croivalve.com