ImmuneOncia Announces Biomarker Results from Phase 1 Clinical Trial of CD47 Antibody at ASCO
ImmuneOncia Announces Biomarker Results from Phase 1 Clinical Trial of CD47 Antibody at ASCO
SEOUL, South Korea--(BUSINESS WIRE)--ImmuneOncia (CEO Heung Tae Kim) announced the results of its solid tumour Phase 1a clinical trial of IMC-002, an anti-CD47 mAb, presented at the American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, USA on June 1, 2024 (local time). These results include updates on the Phase 1a clinical trial outcomes, along with biomarker findings achieved through collaboration with Lunit (CEO; Brandon Suh), a leading provider of AI-powered solutions for cancer diagnostics and therapeutics.
The study, a dose escalation part of Phase 1, enrolled a total of 12 patients across four dose cohorts starting from May 2022. Each patient received IMC-002 at doses of 5, 10, 20, or 30 mg/kg every two weeks. Treatment outcomes revealed stable disease (SD) in 6 out of 12 evaluable patients, demonstrating a disease control rate (DCR) of 50%. Among these, 5 patients had hepatocellular carcinoma, and 1 had breast cancer. Furthermore, 4 patients maintained stable disease for over 6 months, resulting in a clinical benefit rate (CBR) of 33.3%.
AI-powered biomarker analysis, utilizing Lunit SCOPE IO, revealed that the density of CD47-positive macrophages was numerically higher in the CBR group compared to the non-CBR group (71.0/mm² vs. 44.3/mm²), while the density of CD47-positive tumour cells was similar between the two groups. These findings suggest a correlation between the density of CD47-positive macrophages and treatment response, underscoring the potential significance of targeting CD47 in future therapeutic strategies.
Principal investigator Dr. Ho Yeong Lim of Samsung Medical Center stated, "No dose-limiting toxicities (DLTs) were observed across all cohorts, and common adverse events associated with anti-CD47 therapy such as infusion-related reactions, haemolytic anaemia, thrombocytopenia, and neutropenia were not reported, confirming the high safety profile of IMC-002." He added, "Particularly noteworthy is the absence of long-term toxicity in a patient with hepatocellular carcinoma who has been on single-agent IMC-002 treatment for 15 months, demonstrating a favourable prognosis with stable disease and a 20% reduction in tumour size."
CEO Heung Tae Kim of ImmuneOncia commented, "We previously disclosed the high safety and tolerability of IMC-002, along with the recommended Phase 2 dose (RP2D) of 20 mg/kg every 3 weeks, based on interim results presented at the European Society for Medical Oncology (ESMO) in October 2023." He further stated, "With the Phase 1b trial for IMC-002 initiated last November, we anticipate additional efficacy confirmation for IMC-002 in specific solid tumours with high unmet needs."
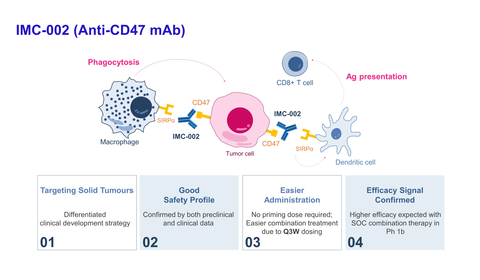
About IMC-002
IMC-002 is an immune-checkpoint-inhibitor targeting the CD47 of macrophages, blocking the 'don't eat me' signal of CD47/SIRPα interaction between cancer cells and macrophages to enhance phagocytosis of cancer cells. IMC-002 is a potential first-in-class and second-generation anti-CD47 mAb demonstrating high safety with no commonly associated adverse events (hemagglutination, thrombocytopenia, neutrophilia, etc.) due to minimal binding to normal cells such as red blood cells.
About ImmuneOncia Therapeutics, Inc.
ImmuneOncia is a South Korean clinical-stage biotech company focused on immuno-oncology, established in 2016. Leveraging expertise in drug development and antibody engineering, ImmuneOncia is dedicated to delivering safe, effective, and novel immunotherapies to oncology patients worldwide. Its portfolio includes a variety of immune checkpoint antibodies such as PD-L1, CD47, LAG3, Bi-specifics, and ADC/AIC.
Contacts
ImmuneOncia Therapeutics, Inc.
Jiwon Heo
+82 2-6283-5098
PR@immuneoncia.com