Rapid Medical™ Completes Successful Ischemic Stroke Procedures with the World’s First Robotic Thrombectomy Device
Rapid Medical™ Completes Successful Ischemic Stroke Procedures with the World’s First Robotic Thrombectomy Device
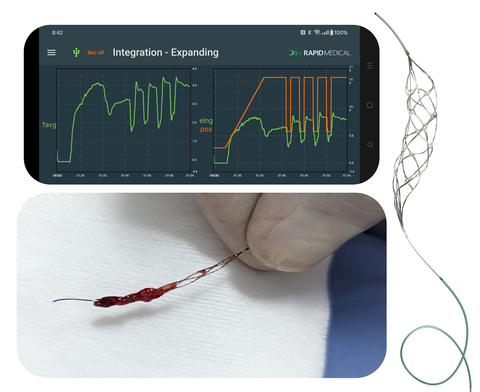
The procedures utilized an AI-powered, robotic TIGERTRIEVER to automate and optimize personalized treatments
YOKNEAM, Israel & DALLAS--(BUSINESS WIRE)--Rapid Medical™, a leading developer of advanced neurovascular devices, announces the first successful robotic thrombectomy in Medellín, Colombia. Two patients were treated with the Robotic TIGERTRIEVER™, the first endovascular thrombectomy device that adapts autonomously to the patient’s anatomy.
“Automating stroke procedures is a significant advancement for the medical field,” emphasizes Dr. Boris Pabón, the endovascular neurosurgeon at Angiosur who performed the first-in-man procedures. “Robotic clot removal achieves a level of precision that surpasses interventional capabilities. The robotic TIGERTRIEVER gives me direct insight into the vessel characteristics and senses what I cannot–truly remarkable.”
With the press of a button, the AI-activated TIGERTRIEVER captures the blockage with active integration. The device’s radial force is then automatically reduced to minimize trauma to the brain as it is retrieved. This builds upon the excellent clinical results and procedure times seen with TIGERTRIEVER’s adjustability, further enhancing precision and streamlining workflow.
“Implementing this technology is a testament to our commitment to expanding treatment for stroke patients worldwide,” affirms Ronen Eckhouse, CEO of Rapid Medical. “We are at the forefront of advancing care—first by providing physicians with active technology and now paving a new pathway to automate procedures with intelligent control.”
About Rapid Medical
Rapid Medical expands what’s possible in neurovascular treatment by pioneering advanced interventional devices that treat ischemic and hemorrhagic stroke. Utilizing proprietary manufacturing techniques, Rapid Medical’s products are remotely adjustable and fully visible. This enables physicians to respond in real time to the anatomy and tailor the approach to every patient for better procedural outcomes. TIGERTRIEVER™ 13, 17, and 21, COMANECI™ and COLUMBUS™/DRIVEWIRE are CE marked and FDA cleared. TIGERTRIEVER XL is also CE marked. More information is available at www.rapid-medical.com.
Contacts
Ronen Eckhouse
+972-72-2503331
ronen@rapid-medical.com