Reflow Medical Completes Enrollment in the DEEPER REVEAL Clinical Trial
Reflow Medical Completes Enrollment in the DEEPER REVEAL Clinical Trial
SAN CLEMENTE, Calif.--(BUSINESS WIRE)--Reflow Medical, Inc., a developer of innovative devices focused on cardiovascular disease, announces completion of enrollment in the DEEPER REVEAL clinical trial (NCT05358353) to evaluate the Reflow Spur™ Stent. Designated a Breakthrough Device by the FDA, the Spur is a unique clinical solution intended to provide stent-like results while leaving no metal behind. The study enrolled 130 patients in over 35 clinical centers in the U.S.
“The steady rate of enrollment, along with the level of support from so many centers, has been impressive. The response reflects the huge unmet need for treatment options for CLTI,” said Dr. S. Jay Mathews, MD, MS, FACC, FSCAI, one of the Lead Principal Investigators and Cath Lab Director at Bradenton Cardiology/Manatee Memorial Hospital in Bradenton, Florida. He continued, “We’re looking forward to next steps in working with the FDA to bring this technology to our patients.”
Dr. Mahmood K. Razavi, MD, FSIR, FSVM, one of the lead PIs and the Director of the Clinical Trials and Research Center at St. Joseph Heart & Vascular Center in Orange, California, commented, “As a physician who has worked with Reflow, I have followed the clinical data generated from the EU trials, which are very promising. I’m looking forward to seeing the final clinical data from the DEEPER REVEAL trial.”
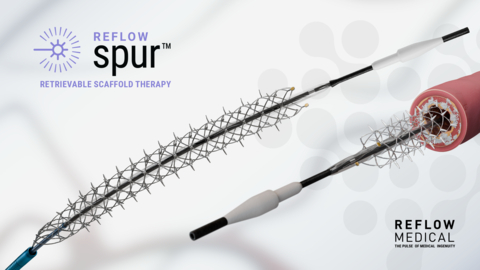
The prospective, single-arm, multicenter study will examine the efficacy and safety of the Spur Stent for the treatment of vascular lesions in the infrapopliteal arteries below the knee. A novel device intended for use in patients with critical limb-threatening ischemia (CLTI), this Retrievable Scaffold Therapy (RST) features a retrievable stent with radially self-expanding spikes that treat the diseased vessel wall without leaving anything behind.
Teo Jimenez, Senior VP of R&D at Reflow Medical, commented on completion of enrollment in the DEEPER REVEAL study: “We want to thank everyone who has partnered with us in completing this enrollment. Our next step is to gather the necessary follow-up data on our path to U.S. approval.”
About Reflow Medical, Inc. Reflow Medical, Inc. focuses on empowering physicians through the design and development of innovative and effective technologies for cardiovascular disease. The Reflow portfolio includes products designed for use in treating cardiovascular disease in the coronary arteries, as well as the peripheral vasculature.
Contacts
Jennifer Carlyle
jcarlyle@reflowmedical.com
949-481-0399