Valar Labs Announces Validation of First Histology-based Test to Predict Response to BCG in Bladder Cancer
Valar Labs Announces Validation of First Histology-based Test to Predict Response to BCG in Bladder Cancer
–Vesta, an Innovative New Diagnostic Test to Guide First-Line Treatment Decisions–
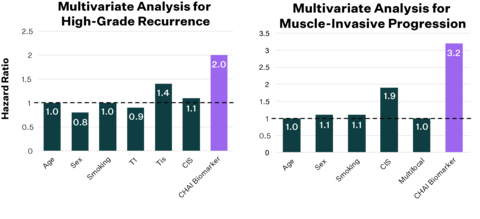
The CHAI biomarkers offer independent prognostic capabilities for high-grade recurrence and muscle-invasive progression in bladder cancer. Additionally, they identify patients less likely to benefit from BCG therapy. Validated across more than 1,000 patients and 12 centers, these biomarkers, available through the Vesta test, can enhance first-line treatment decision-making for non-muscle invasive bladder cancer. (Graphic: Business Wire)
PALO ALTO, Calif.--(BUSINESS WIRE)--Valar Labs, the developer of computational histology AI tests for predicting response to cancer therapies, announced new data today which is being presented on Saturday, May 4, 2024 at the 2024 Annual Meeting of the American Urological Association (AUA) as a podium presentation (PD30-03). The study demonstrated that the first-ever computational histology AI (CHAI) assay with biomarkers for response prediction in bladder cancer can help urologists make more informed treatment decisions for their patients. The biomarkers were successfully validated across 1000+ patients and 12 centers on four end-points, making it the largest validation study of its kind in bladder cancer.
“Our study demonstrates that the CHAI biomarkers can identify patients with high-risk non-muscle invasive bladder cancer for whom BCG therapy is likely to be ineffective, across a diverse set of patients,” said Yair Lotan, M.D., Professor of Urology, Chief of Urologic Oncology, and holder of the Jane and John Justin Distinguished Chair in Urology, in Honor of Claus G. Roehrborn, M.D. at UT Southwestern Medical Center. “It’s crucial to spare non-responding patients from the unnecessary toxicity of ineffective treatments and to allocate BCG more effectively, especially amidst the current nationwide BCG shortage.”
Data showed that patients with biomarker present had 3x elevated risk of progression to muscle invasive disease within 3 years. Additionally, patients with biomarker present had 2x elevated risk of non-response to Bacillus Calmette-Guerin (BCG) as well as high-grade recurrence within 6 and 24 months. The biomarkers demonstrated significant predictive ability across all subgroups and provided independent prognostic capabilities beyond common clinical risk factors.
“Accurately identifying patients with high-risk non-muscle invasive bladder cancer that will not respond to BCG is essential so they can receive the treatment that is best suited for them,” said Stephen B. Williams, M.D., Associate Chief Medical Officer, Chief of Urology, Director of Urologic Oncology at The University of Texas Medical Branch. “There are multiple therapies that are coming to market and some of which are already being used, such as the combination of gemcitabine and docetaxel, that should be considered for patients with CHAI biomarkers present.”
The CHAI biomarkers consists of 600 features measuring tumor and tumor micro-environment characteristics that include features indicative of tumor aggressiveness, nuclear and cellular pleomorphism, mitotic activity, and immune infiltration.
“Bladder cancer presents a significant challenge, affecting over 80,000 individuals annually and until now, it has been markedly underserved by advancements in precision medicine,” said Anirudh Joshi, co-founder and CEO of Valar Labs. “With response rates to BCG treatment as low as 50%, physicians have long sought tools like our CHAI biomarkers to better manage patient care. We are deeply committed to addressing this deficiency by advancing precision diagnostics in bladder cancer.”
“We are incredibly excited to have completed the validation of these biomarkers for predicting response to the standard of care therapy in high-risk non muscle-invasive bladder cancer. We are eager to make this transformative test available to every patient and physician across the country,” said Damir Vrabac, co-founder and COO of Valar Labs. “A test that identifies patients unlikely to respond to BCG will empower physicians to tailor treatment pathways more effectively and enhance patient outcomes.”
“Computational histology AI biomarkers are extremely powerful and we are just scratching the surface of what this technology is capable of,” said Viswesh Krishna, co-founder and CTO of Valar Labs. “We are proud to be at the forefront of this groundbreaking shift in diagnostics, uncovering new sets of biomarkers based on histology. We are able to visualize these biomarkers and gain a better understanding of how tumor morphology correlates to treatment response.”
About Valar Labs:
Valar Labs is a precision medicine company developing computational histology AI tests for predicting response to cancer therapies. Our goal is to provide every patient and physician the ability to select the best treatment based on the patient's tumor biology. Our bladder cancer test, Vesta, predicts which patients are unlikely to benefit from BCG therapy and provides detailed risk stratification for recurrence and progression. Vesta is clinically available through our CLIA-certified laboratory in Houston, Texas and can be ordered online. For more information, please visit www.valarlabs.com and follow us on LinkedIn and Twitter.
Contacts
Media Relations
+1 (888) 862-0232
media@valarlabs.com