Aerin Medical Announces Establishment of a New CPT® Code for Endoscopic Destruction of the Posterior Nasal Nerve Using Radiofrequency Ablation
Aerin Medical Announces Establishment of a New CPT® Code for Endoscopic Destruction of the Posterior Nasal Nerve Using Radiofrequency Ablation
Procedure Regularly Used by ENT Physicians Treating Chronic Rhinitis Patients
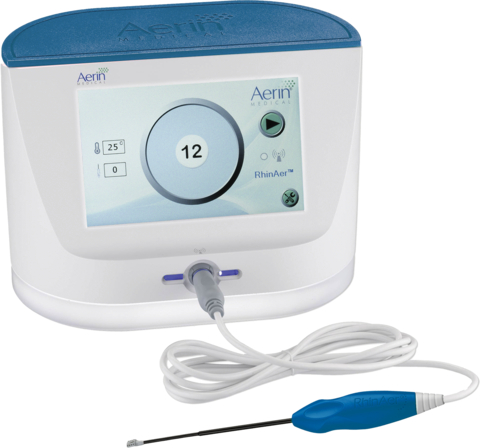
A new Category I CPT code for endoscopic destruction of the posterior nasal nerve using radiofrequency ablation will be effective January 1, 2024. The new code describes the procedure that ENT surgeons perform using RhinAer. RhinAer is a non-invasive, temperature-controlled radiofrequency technology that durably treats the causes of rhinorrhea (runny nose), post-nasal drip and congestion associated with chronic rhinitis in a single session. (Photo: Business Wire)
MOUNTAIN VIEW, Calif.--(BUSINESS WIRE)--Aerin Medical Inc., a company dedicated to providing Ear, Nose and Throat (ENT) physicians with non-invasive solutions for the treatment of chronic nasal conditions, announced that the American Medical Association (AMA) Current Procedural Terminology (CPT®) Editorial Panel accepted the application for a new Category I CPT code to report endoscopic destruction of the posterior nasal nerve using radiofrequency (RF) ablation. This information was released by the AMA in the Summary of Panel Actions from the September CPT Editorial Panel meeting. The new code will become effective on January 1, 2024, and describes the procedure performed by ENT physicians when treating their patients with Aerin Medical’s RhinAer®. The RhinAer Stylus precisely applies RF energy to overactive nerves in the back of the nose, reducing chronic rhinitis symptoms.
“A new CPT code is an important milestone for ENT physicians and their patients, enabling appropriate valuation and improving access to this transformative solution that can be readily performed in the physician office setting,” said Matt Brokaw, CEO of Aerin Medical. “We appreciate the otolaryngology society leadership and physician investigator commitment to generating robust clinical evidence for the RF approach, including the longest-term published randomized controlled trial data available.”
The company recently announced the launch of a next-generation RhinAer Stylus designed to provide physicians with improved visualization, as well as easier access and tissue apposition, especially in patients with narrow nasal airways.
About RhinAer
Using temperature-controlled, radiofrequency technology, RhinAer features a thin, wand-like stylus that is inserted via the nostril to deliver precise therapeutic benefits, while sparing surrounding tissues. RhinAer directly disrupts the posterior nasal nerve (PNN) that triggers excessive mucus production, treating an underlying cause of chronic rhinitis. RhinAer provides ENT physicians with a comprehensive solution for the treatment of chronic rhinitis, addressing sources of rhinorrhea (runny nose) and congestion. RhinAer initially received FDA 510(k) clearance in December 2019 and CE Mark in 2020. For more information, visit www.RhinAer.com.
About Aerin Medical
Aerin Medical is a privately held, venture-backed company, with U.S. offices in California and Texas. Aerin’s mission is to provide ENT physicians with non-invasive solutions for the treatment of chronic nasal conditions. The company’s products, VivAer® for nasal airway obstruction and RhinAer® for chronic rhinitis, leverage Aerin’s proprietary temperature-controlled technology, which allows ENT physicians to reliably improve patients’ symptoms with in-office procedures performed with local anesthetic. More than 60,000 patients have been treated with Aerin Medical products to date. For more information, please visit www.aerinmedical.com and follow Aerin Medical on Facebook, Twitter, Instagram, LinkedIn and YouTube.
Contacts
Keshia Cain
573-517-1593
keshia@merrymancommunications.com