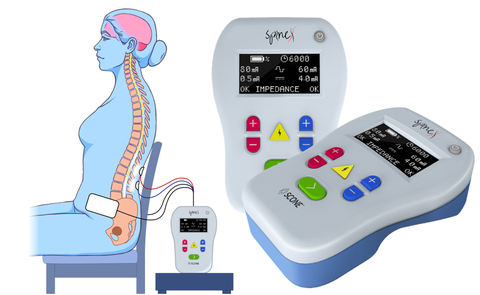
SpineX Announces First Patient Enrollment in SCONE Clinical Trial
SpineX Announces First Patient Enrollment in SCONE Clinical Trial
SCONE trial is assessing safety and efficacy of the SCONE™ device for Neurogenic Bladder
LOS ANGELES--(BUSINESS WIRE)--SpineX Inc., a clinical stage bioelectric medtech company, today announced that the first patient has been enrolled in a pivotal trial evaluating the SCONE™ Device. The pivotal trial, known as SCONE trial, will evaluate the safety and effectiveness of the SCONE™ device for the treatment of Neurogenic bladder. This is the first large-scale pivotal trial testing a non-invasive spinal neuromodulation technology for the treatment of neurogenic bladder. The first patient was enrolled at Rancho Research Institute, the research arm of the prestigious and historic Rancho Los Amigos National Neurorehabilitation Center, Downey, California (https://www.ranchoresearch.org/).
Neurogenic bladder is the most common comorbidity that people suffer after paralysis, and has the largest impact on their quality of life. Surveys suggest that individuals living with paralysis rate improvement in bladder, bowel and sexual function as a higher priority than restoration of ambulation and even resolution of chronic pain. “For a person in a wheelchair, the inability to walk is the most obvious functional loss, but the impact on quality of life due to neurogenic bladder is unparalleled,” said Dr. Evgeniy Kreydin, MD, Co-Founder, SpineX Inc., and Assistant Professor at University of Southern California, Los Angeles.
Impact of neurogenic bladder extends beyond the inability to void as needed or the necessity for lifelong daily repetitive catherizations to empty their bladder. People living with neurogenic bladder have to deal with lost sensation of bladder fullness, low bladder capacity, frequent urination cycles during the day and night, and live with a constant fear of uncontrolled urine leakage. SCONE™ therapy is designed to treat each of these symptoms, thus having a global impact on quality of life.
“The initiation of the SCONE trial is an important milestone in bringing the world’s first non-invasive treatment modality for neurogenic bladder to market,” said Dr. Parag Gad, PhD, Chief Executive Officer of SpineX Inc. “We are committed to transforming bladder management into a catheter free and leak free world.”
About SpineX Inc.
SpineX Inc., a clinical stage bioelectric medtech company developing Noninvasive Spinal Neuromodulation devices as a platform technology. SCONE™ and SCiP™ are two FDA-designated Breakthrough Devices being developed by SpineX for the treatment of adults with Neurogenic Bladder and Children with Cerebral Palsy respectively. To learn more about SpineX and the ongoing trial, visit www.spinex.co or follow @spinex_inc on social media.
Contacts
Parag Gad, parag@spinex.co