FDA Clearance Brings RefleXion Closer to Expanding Cancer Treatment Market
FDA Clearance Brings RefleXion Closer to Expanding Cancer Treatment Market
X1 Machine Rotates up to 60 Times Faster than Other Linear Accelerators for Precise Dose Delivery to Tumors
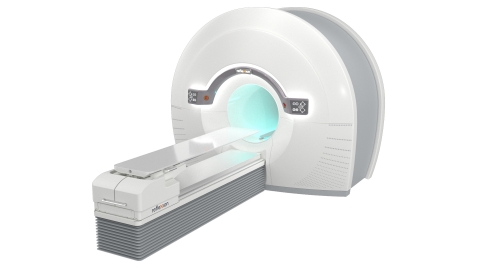
The RefleXion™ X1 is the only machine that combines high-quality CT imaging with a linear accelerator for better tumor localization. The groundbreaking design rotates up to 60 times faster than other linear accelerators and modulates the radiation dose from 100 points per beam station for precise dose delivery. (Photo: Business Wire)
HAYWARD, Calif.--(BUSINESS WIRE)--RefleXion Medical, a therapeutic oncology company pioneering the use of biology-guided radiotherapy (BgRT)* to treat all stages of cancer today announced it has received marketing clearance from the U.S. Food & Drug Administration (FDA) for stereotactic body radiotherapy (SBRT), stereotactic radiosurgery (SRS) and intensity modulated radiotherapy (IMRT) for its RefleXion™ X1 machine.
“We are at the forefront of an enormous change in expanding the use of radiotherapy from a treatment solely for early-stage cancer patients to an entirely new group of patients who need it most, those with advanced stage cancer,” said Todd Powell, CEO and president of RefleXion. “This initial marketing clearance of our RefleXion X1 machine is a steppingstone on the path to our goal of providing BgRT as a novel treatment modality that will expand the overall radiotherapy market significantly.
“We have been preparing for commercial operations in the brand new factory we opened last year and expect to begin machine shipments within the next quarter,” continued Powell.
The RefleXion X1 is the only machine that combines high quality computed tomography (CT) imaging, known as fan-beam CT, with a linear accelerator to reduce motion artifacts that can inhibit accurate targeting of the radiation dose to a patient’s tumor. Its groundbreaking design rotates up to 60 times faster than other linear accelerators and modulates dose delivery from 100 points per beam station. These combined improvements may reduce the side effects of radiotherapy by allowing radiation oncologists to better localize the tumor, reduce patient setup errors and precisely deliver dose to complex tumor targets while avoiding nearby normal structures.
“Transitioning this company from a research effort to what is, as of today, a commercial entity has been a thrilling 10-year journey,” said Sam Mazin, Ph.D., founder and CTO of RefleXion. “Along the way, RefleXion has become an organization supported by dedicated and brilliant people in every corner, including our clinical partners. We will celebrate this milestone and then quickly turn our sights to bringing our biology-guided radiotherapy to market.”
BgRT, a novel treatment modality under development, uses the biological signature of a tumor to characterize its movement and to deliver a precisely tracked therapeutic radiation dose to the tumor. The RefleXion X1 machine with BgRT is designed to overcome the technical limitations that currently restrict radiotherapy to one or two tumors. Once developed, RefleXion will scale BgRT to treat all visible tumors, even those that move rapidly due to involuntary processes such as breathing or digestion, in the same treatment session.
About RefleXion Medical
RefleXion is a privately-held company developing the first biology-guided radiotherapy system, a significant change in strategy from single tumor therapy to the ability to one day treat multiple tumors in the same treatment session in cancers that have metastasized. Currently, the RefleXion machine is cleared for the delivery of stereotactic body radiotherapy (SBRT), stereotactic radiosurgery (SRS) and intensity modulated radiotherapy (IMRT). The company is also developing BgRT, which incorporates positron-emission tomography (PET) imaging data to enable tumors to continuously signal their location. The BgRT technology will synchronize these data with the linear accelerator to direct radiotherapy to tumors with subsecond latency.
RefleXion is backed by premier investment firms TPG Growth/The Rise Fund, Sofinnova Partners, KCK Group, Venrock, T. Rowe Price and global pharmaceutical leaders Pfizer Venture Investments and Johnson & Johnson Innovation, JJDC Inc.
*The RefleXion X1 BgRT capability requires 510(k) clearance and is not available for sale.
Contacts
RefleXion
Amy Cook
acook@reflexion.com
925.200.2125