Orthofix Announces Agreement to Acquire FITBONE Limb Lengthening System
Orthofix Announces Agreement to Acquire FITBONE Limb Lengthening System
Transaction expands limb reconstruction portfolio
LEWISVILLE, Texas--(BUSINESS WIRE)--Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company focused on musculoskeletal healing products, today announced an asset purchase agreement with Wittenstein SE, a privately-held German-based company, to acquire assets associated with the FITBONE® intramedullary lengthening system for limb lengthening of the femur and tibia bones. Additionally, the transaction brings other potential applications of the technology, which are in preliminary development, including the FITSPINE® system for early onset scoliosis. Terms of the agreement include $18 million in cash closing consideration and a manufacturing supply contract with Wittenstein SE.
“Founded 40 years ago as an extremity fixation company, Orthofix has a long history of cutting-edge solutions to treat patients born with limb discrepancies,” said Orthofix President and Chief Executive Officer Jon Serbousek. “The addition of the FITBONE intramedullary lengthening system further rounds out our limb reconstruction offerings and is well aligned with our strategy of investing in innovative products to drive growth within our core businesses.”
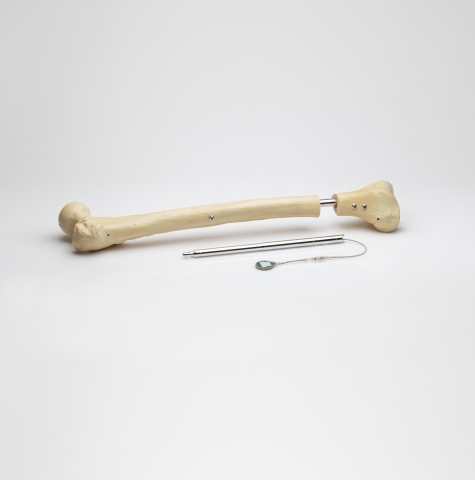
The FITBONE system consists of an intramedullary lengthening nail that is surgically implanted in the bone through a minimally invasive procedure, an external control set that manages the distraction process, and the FITBONE app that supports the patient throughout the limb lengthening treatment. Over 3,500 cases in more than 15 countries have been performed with the FITBONE system.
With the addition of the FITBONE system, Orthofix becomes the only orthopedic company that offers a comprehensive portfolio of both internal and external fixation solutions for limb reconstruction. The FITBONE system will augment Orthofix’s current deformity and limb reconstruction offerings that include the TL-HEX™ computer-assisted ring fixation system for external limb lengthening and the eight-Plate Guided Growth System™ for correcting angular growth deformities in pediatric patients. To learn more about Orthofix’s dedication to helping surgeons and limb deformity correction patients, please visit JuniOrtho.club.
“We are proud that we could bring a product such as the FITBONE intramedullary lengthening system to the market to help people in need of limb lengthening,” said Dr. Bertram Hoffmann, Chief Executive Officer of Wittenstein SE. “It has long been our goal to increase global availability. We are pleased that through Orthofix, more surgeons and their patients will have access to this innovative technology that can make a huge difference in an individual’s quality of life.”
The FITBONE intramedullary lengthening system is available in the U.S. under a U.S. Food and Drug Administration 510(k) clearance and in European Countries under a CE Mark approval. The acquisition is anticipated to close by the end of the first quarter of 2020, subject to customary closing conditions. Orthofix does not expect the transaction to be material to 2020 net sales. More information regarding this announcement will be made available during the Company’s fourth quarter and fiscal year 2019 financial results call.
About Orthofix
Orthofix Medical Inc. is a global medical device company focused on musculoskeletal products and therapies. The Company’s mission is to improve patients' lives by providing superior reconstruction and regenerative musculoskeletal solutions to physicians worldwide. Headquartered in Lewisville, Texas, Orthofix’s spine and orthopedic extremities products are distributed in more than 70 countries via the Company's sales representatives and distributors. For more information, please visit www.orthofix.com.
About Wittenstein SE
With around 3000 employees worldwide and sales of €436.4 million in 2018/19, Wittenstein SE enjoys a reputation for innovation, precision and excellence in the field of mechatronic drive technology – not just in Germany but internationally. The group comprises six pacesetting Business Units with separate subsidiaries for servo gearboxes, servo actuator systems, medical technology, miniature servo units, innovative gearing technology, rotary and linear actuator systems, nanotechnology and electronic and software components for drive technologies. Through its 60 or so subsidiaries and agents in approximately 40 countries, Wittenstein SE (www.wittenstein.de) is additionally represented in all the world's major technology and sales markets.
Forward-Looking Statements
This communication contains certain forward-looking statements under the Private Securities Litigation Reform Act of 1995. These forward-looking statements, which may include, but are not limited to, statements concerning the estimates, projections, financial condition, results of operations and businesses of Orthofix and its subsidiaries, Wittenstein SE and their respective companies’ product portfolios, are based on Orthofix management's current expectations and estimates and involve risks and uncertainties that could cause actual results or outcomes to differ materially from those contemplated by the forward-looking statements.
The forward-looking statements in this release do not constitute guarantees or promises of future performance. Factors that could cause or contribute to such differences may include, but are not limited to risks, including the possibility that the deal might not close, difficulties commercializing products and integrating the product into Orthofix’s business, inaccuracies in Orthofix’s estimates and projections of future product sales, including the current and future size of the worldwide and U.S. limb reconstruction market, FDA and regulatory approval risks, and other risks described in the "Risk Factors" section of our 2018 Annual Report on Form 10-K, as well as in other reports that we file in the future. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. The Company undertakes no obligation to update or revise the information contained in this press release.
Contacts
Mark Quick
Investor Relations
Tel 214 937 2924
markquick@orthofix.com
Denise Landry
Media Relations
Tel 214 937 2529
deniselandry@orthofix.com