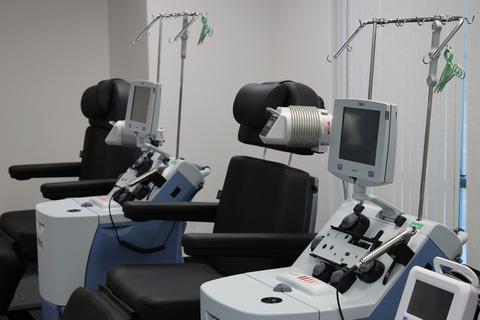
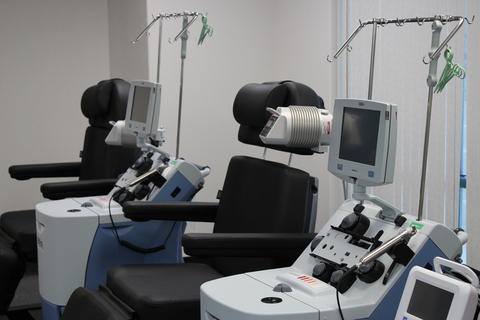
SPRINGFIELD, Mo.--(BUSINESS WIRE)--QPS Holdings, LLC announced today that their Springfield campus, QPS Missouri, has launched a new Cell Therapy business unit. The unit’s first achievement is the opening of a new Leukopak collection and blood product processing center. This facility is designed to meet the exponentially growing demand for blood products to support cell and gene therapy work. QPS is a full service CRO that’s dedicated to supporting drug development from conceptualization through to commercial launch. This facility is providing critical raw materials for the research and development of cell therapies but will soon be supporting clinical and commercial cell therapy companies and patients. In addition, this new facility will support the local economy by providing stipends to blood product donors.
To build out the facility and ensure the success of this new venture, QPS has hired Tia Hexom, PhD, (Senior Director of Cell Therapy Services and Manufacturing). With over a decade of experience in the cell therapy field, Hexom is excited to be building cell therapy capabilities in the Midwestern United States.
QPS Missouri is a division of QPS Holdings LLC, a global Clinical Research Organization (CRO) that conducts studies on behalf of pharmaceutical and biotech companies for the development of new and existing pharmaceutical products. This new Leukopak and Cell Therapy products donation and processing center will be housed in the same building as the new, state-of-the-art Screening and Recruitment Center that opened in July 2022, located at 2025 W. Sunshine Street, Springfield, MO. Study participants and blood product donors are paid a stipend for their participation in these Clinical Research activities. These stipends benefit the study participants and donors and, therefore, the local economy.
“Our participants and donors are a key part of the success of QPS in Springfield, Missouri and they continue to make a difference by helping advance drug development research,” states Brendon Bourg, Vice President, Early Phase Clinical/Head of Administration QPS Missouri. “To have the opportunity and ability to provide this type of supplemental income to our local community, while providing a valuable service to our pharmaceutical and biotech clients is something we (QPS) are honored to be a part of.”
Located in Springfield, QPS Missouri has been conducting clinical research studies for over 30 years, with the help of more than 50,000 study participants. In addition to the new Cell Therapy facilities, the Springfield campus houses five independent clinics with more than 240 beds, a pharmacy with a retention area, local and central CAP/CLIA safety lab, a clinical trial kit processing area, and a 2,500 square foot negative pressure room.
QPS has always been on the front line of clinical research in all areas of pharmaceutical and biotech drug development. Since opening its doors in Springfield in 1994, QPS Missouri has conducted more than 2,000 Phase I FDA-regulated studies and paid over $50 million to local participants and donors.
ABOUT QPS HOLDINGS, LLC
QPS is a global, full-service, GLP/GCP-compliant contract research organization (CRO) delivering the highest grade of discovery, bioanalysis, preclinical and clinical drug development services. Since 1995, QPS has grown from a small bioanalysis shop into a full-service CRO with 1,100+ employees in the US, Europe, and Asia. Today, QPS offers expanded pharmaceutical contract R&D services with special expertise in pharmacology, DMPK, toxicology, bioanalysis, translational medicine, cell therapy products (such as Leukopaks and PBMCs), clinical trials, and clinical research services. An award-winning leader focused on bioanalysis and clinical trials, QPS is known for proven quality standards, technical expertise, a flexible approach to research, client satisfaction, turnkey laboratories, Phase I clinical facilities, and multi-site clinical research services. Through continual enhancements in capacities and resources, QPS stands tall in its commitment to delivering superior quality, skilled performance, and trusted service to its valued customers. For more information, visit www.qps.com or email info@qps.com.