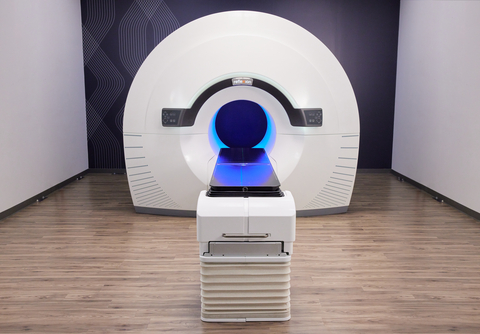
HAYWARD, Calif.--(BUSINESS WIRE)--RefleXion® Medical, an external-beam theranostic oncology company, today announced initiation of the BIOGUIDE-X2 clinical study to expand indications for SCINTIX® biology-guided radiotherapy at Hackensack Meridian John Theurer Cancer Center, a research partner with Georgetown’s Lombardi Comprehensive Cancer Center, an NCI-designated comprehensive cancer center.
The company also announced key updates from the Centers for Medicare and Medicaid (CMS) Services. Starting in 2025, CMS will provide broader reimbursement for SCINTIX therapy, including new codes for freestanding cancer centers (FSC) and professional reimbursement for physicians in FSC and hospital outpatient departments.
“The achievement of these milestones sets the stage for substantial expansion in both the indications for and access to SCINTIX therapy," said Sean Shirvani, M.D., M.P.H., chief medical officer at RefleXion. "Given that 40% of American cancer patients receive radiation therapy in freestanding centers1, securing reimbursement for these facilities was imperative, as it eliminates payment barriers for providers adopting new technologies and grants patients access to innovative treatment options.”
The Medicare updates simplify and expand reimbursement, allowing approximately 400,000 additional patients,2 including those suffering from metastatic disease, access to SCINTIX therapy. These changes also support physicians by introducing a professional payment coding mechanism, acknowledging the increased effort necessary to facilitate the widespread adoption of this groundbreaking technology.
“We are also buoyed by the initiation of the BIOGUIDE-X2 clinical study, designed as a multi-cohort study for patients with cancers in the liver and abdomen as the first cohorts. We are grateful to our clinical champions at all our sites for their assistance in designing and implementing this study,” continued Shirvani.
The BIOGUIDE-X2 study is intended to expand SCINTIX therapy from current lung and bone indications to include patients with liver, gallbladder, pancreatic, pelvic, head-and-neck, and other solid tumor cancers. The multi-site, multi-cohort study will gather data at six clinical sites, including Hackensack Meridian John Theurer Cancer Center in New Jersey, over the next several months.
About RefleXion Medical
RefleXion Medical, headquartered in Hayward, Calif., develops and commercializes SCINTIX biology-guided radiotherapy, a groundbreaking treatment that uses a single radiotracer injection to transform cancer cells into real-time biological beacons to control external-beam radiotherapy delivery to single or multiple tumors. SCINTIX therapy is indicated for use in FDG-guided treatment of lung and bone tumors and is being studied for broader indications. In addition, the company offers the RefleXion® X1 system for conventional radiotherapy for all solid tumors located anywhere in the body. More information may be found at https://reflexion.com/.
__________________________
1 https://docs.house.gov/meetings/IF/IF14/20171108/106599/HHRG-115-IF14-Wstate-KavanaghB-20171108.pdf
2 Ibid