SHANGHAI--(BUSINESS WIRE)--Xgene Pharmaceutical announced additional insights into the positive results of a multiple-center, placebo-controlled, dose-ranging Phase 2b study (registration number: NCT06017999) in patients undergoing bunionectomy, evaluating the safety and efficacy of the XG005 oral tablet on acute pain.
XG005 is a non-opioid, new chemical entity with dual mode of action in targeting both nociceptive and neuropathic pain signals. The placebo, 750 mg or 1250 mg of XG005, was administered orally twice a day (bid) for 72 hours to patients undergoing bunionectomy. The study was collaborated with Evolution Research Group and Lotus Clinical Research at multiple sites in the USA. A total of 450 subjects were enrolled into the trial with 1:1:1 randomization ratio. The drug was well tolerated with an acceptable safety profile. There were no drug-related serious adverse events.
The primary and the key secondary efficacy endpoints of SPI over 48 hours for the 1250 mg and 750 mg dose group versus placebo achieved statistically significant difference, respectively with 1250 mg dose providing significantly more analgesia than 750 mg.
Other efficacy endpoints are briefly listed below:
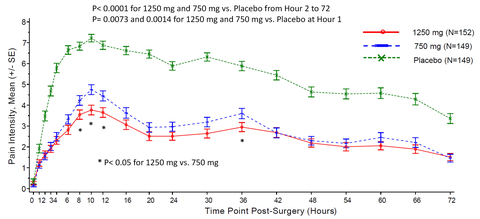
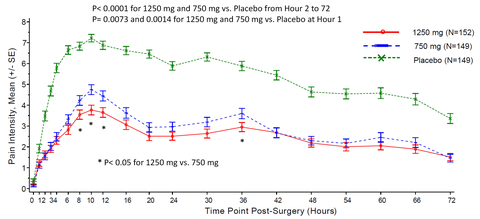
- The pain intensity score was statistically lower in both active arms compared to placebo over 72 hours post-surgery (P< 0.0001); the maximum pain was mild (< 4.0 NRS) in severity in the high dose arm, in contrast to up to severe pain (> 7.0 NRS) score in the placebo arm during the same period (see figure).
- The time to first use of rescue medication was delayed up to 8-fold in the high dose arm compared with placebo, the median times to first use of rescue medication for high dose, low dose and placebo were 31.47, 12.24 and 4.03 hours, respectively, with Hazard Ratios of 0.18 and 0.24 (P< 0.0001).
- Total opioid consumption over 72 hours was significantly less in active arms than in placebo (P< 0.0001), with Morphine Equivalent Dose (MEQ) of 6.72, 9.38 and 23.86/24.68 mg for the high, low dose of XG005 and placebo, respectively.
- Total acetaminophen use (rescue medication) over 72 hours was significantly less in the active arms than in the placebo (P< 0.0001), with means of 1586.03, 1975.89 and 4812.7/4892.74 mg for the high dose, low dose of XG005 and placebo, respectively.
- Patient Global Assessment (PGA) on pain control was statistically greater in XG005 arms than in the placebo arm over 72 hours (P< 0.0001).
- Patients’ sleep post-surgery was significantly better in the XG005-treated arms compared with placebo as assessed with Sleep Interference Score (P< 0.0001).
“I have not seen any analgesics showing such great efficacy in well-controlled multiple center trials. Mean pain severity was mild at the most in high dose group versus severe in placebo group. The amount of rescue medication used, including opioids, was less than 1/3 in the high dose arm compared with placebo. The standard effect sizes for the primary (high dose) and the key secondary (low dose) efficacy endpoints are 1.55 and 1.28, respectively. This compelling efficacy of XG005 overwhelmingly distinguishes it from other analgesics and we are extremely encouraged to expedite the development for early delivery to patients.” said Leon Jiang, Chief Medical Officer at Xgene Pharmaceutical.
“There is a substantial need for more efficacious, safer, non-opioid treatment for acute pain, as many patients are unable to get sufficient relief with currently available medicines due to intolerable side effects. We are pleased that the results from this trial showed the excellent therapeutic effect of XG005, with the potential to be a better treatment option compared to current therapies,” said Gene Hsu, CEO of Xgene. “Xgene is proud to continue its efforts on this unique product, moving forward with great confidence.”
About Acute Pain
Acute pain is defined as pain lasting less than 3 months. In 2023, the acute pain market was valued at approximately USD 50.03 billion and project to reach around USD 78.36 billion by 2032. Due to limited non-opioid treatment options, there is an unmet need in acute pain management to improve the patient experience and reduce the economic and societal burden. (Acute Pain Market Size, Share, Trends & Report | 2032)
About XG005
XG005 is a novel molecule targeting two distinct pain pathways: an anti-nociceptive and an anti-neuropathic. It is potentially first-in-class oral treatment that is in parallel development for treatment of acute and chronic pain conditions. Due to its dual mechanism of action, XG005 is potentially able to provide greater inhibition of the pain signal transmission achieving better analgesic outcomes while providing good safety profile.
A positive phase 2b trial results of XG005 treatment in chronic osteoarthritis pain was recently reported, and a Phase 2 trial of XG005 in cancer-induced bone pain is currently ongoing in Taiwan and Mainland of China.
About Xgene Pharmaceutical: Transforming Medicine for Better Lives
At Xgene, we apply new clinical findings, science, and innovative technology to bring therapies to patients to extend and significantly improve their lives. We strive for quality, safety and value in our new products. Our goal is to deliver the world's best medicine to patients worldwide. Xgene’s drug discovery areas include pain and CNS conditions focusing research on finding treatments and cures for unmet medical needs. We collaborate with health care providers, governments, and local communities to identify patient needs and deliver reliable, affordable medicines around the world. Visit our website at www.xgenepharm.com.