SAN FRANCISCO--(BUSINESS WIRE)--Novotech, the global full-service clinical Contract Research Organization (CRO) that partners with biotech companies to accelerate the development of advanced and novel therapeutics, has released an in-depth report "COVID-19: Global Clinical Trial Landscape 2024." This report delivers a robust analysis of ongoing and completed COVID-19 clinical trials, therapeutic strategies, and funding trends, offering essential insights for researchers and biotech leaders in the evolving COVID-19 research landscape.
The COVID-19 pandemic has led to significant advancements in clinical research and therapeutic innovation. Novotech’s report offers an authoritative analysis of global trial distribution, patient recruitment trends, and emerging therapeutic strategies, drawing on over 5,000 clinical trials conducted since 2019 across Asia-Pacific, North America, Europe, and beyond.
Key Findings in the Report:
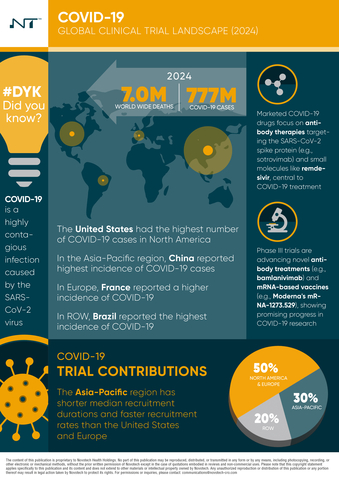
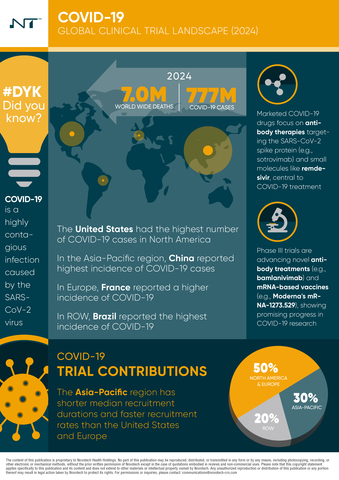
- Epidemiology and Incidence: As of 2024, COVID-19 has resulted in approximately 777 million cases and over 7 million deaths globally, with significant case burdens in regions like Europe, the Americas, and the Western Pacific.
- Global Trial Trends: The report reveals that Asia-Pacific leads COVID-19 clinical trial activity with 34% of trials, followed by North America (25%) and Europe (22%). Key countries, including the United States, India, and China, have played a central role in advancing COVID-19 research.
- Therapeutic Innovations: Evolving strategies now extend beyond vaccines to include targeted antivirals, immune modulators, and monoclonal antibodies each addressing different stages and severities of the disease. These advancements highlight the importance of a diversified approach to COVID-19 management.
- Funding Landscape: Public and venture capital funding has been instrumental in advancing COVID-19 research. Between 2019 and 2023, the U.S. and China received the highest funding, enabling significant progress in the development and deployment of critical COVID-19 treatments.
A Look Ahead: Opportunities and Threats
The report’s SWOT analysis explores both opportunities and challenges within COVID-19 drug development. While booster and variant-specific vaccines, as well as mRNA technology advancements, provide avenues for continued progress, threats such as emerging variants, regulatory challenges, and vaccine hesitancy remain critical considerations.
Download your copy of the "COVID-19: Global Clinical Trial Landscape 2024" report here.
About Novotech Novotech-CRO.com
Founded in 1997, Novotech is a global full-service clinical Contract Research Organization (CRO) focused on partnering with biotech companies to accelerate the development of advanced and novel therapeutics at every phase.
Recognized for its industry-leading contributions, Novotech has received numerous prestigious awards, including the Frost &Sullivan 2024 Global Biotech CRO of the year award, Clinical Trials Arena Excellence Awards 2024, 2024 Employer of Choice, 2024 Great Place to Work in the US, 2024 Brandon Hall Gold Award, CRO Leadership Award 2023, the Asia Pacific Cell & Gene Therapy Clinical Trials Excellence 2023, the Asia-Pacific Contract Research Organization Company of the Year Award since 2006.
The Company offers a comprehensive suite of services including laboratories, Phase I facilities, drug development consulting, regulatory expertise, and has experience with over 5,000 clinical projects, including Phase I to Phase IV clinical trials and bioequivalence studies. With a presence in 34 office locations and a dedicated team of 3,000+ professionals worldwide, Novotech is a trusted end-to-end strategic partner of choice.
For more information or to speak to an expert team member visit www.Novotech-CRO.com