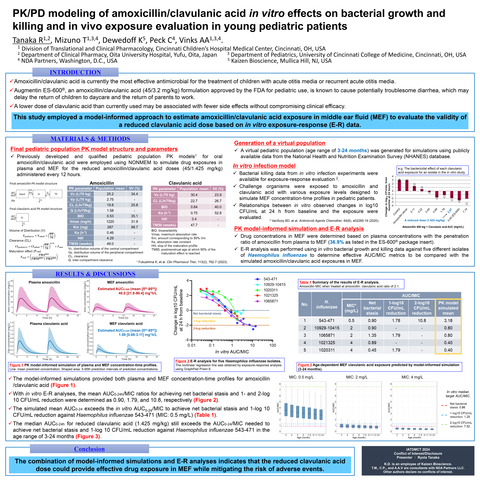
PHILADELPHIA--(BUSINESS WIRE)--Kaizen Bioscience, Co. (‘Kaizen’) a biopharmaceutical company focused on developing & commercializing assets within Infectious Diseases is pleased to announce plans for its first NDA submission in 1H 2025. We would like to congratulate Ryota Tanaka for receiving the https://iatdmct.org/ (International Association of Therapeutic Drug Monitoring and Clinical Toxicology) 2024 BEST TDM Poster Award for the presentation of PK/PD modeling of amoxicillin/clavulanic acid in vitro effects on bacterial growth and killing and in vivo exposure evaluation in young pediatric patients1. “On behalf of Kaizen Bioscience, we would like to thank, Ryota Tanaka, Sander Vinks and Tomoyuki Mizuno with NDA Partners and the individuals at Cincinnati Children’s Hospital Medical Center for their support in developing such an innovative model which anchored the model-informed development of Kaizens PK trial and confidence in our lead program,” says Keith Dewedoff, Co-Founder & Executive Chair, Kaizen Bioscience.
The model demonstrates that a lower dose of clavulanate could provide effective drug exposure in middle ear fluid while mitigating the risk of adverse events commonly found with the current Augmentin ES- 600®, amoxicillin/clavulanate formulation, without compromising clinical efficacy.
Next to the common cold, acute otitis media (AOM) is the most frequently diagnosed illness in children in the United States and the most cited indication for antimicrobial treatment.1 Amoxicillin/clavulanate is currently the most effective antimicrobial for the treatment of children with acute otitis media or recurrent acute otitis media. While the trend over time has been to reduce the total dose of clavulanate in order to lower the incidence of GI related issues and still maintain efficacy against non-susceptible S. pneumoniae, H. influenzae and M. catarrhalis, the current marketed pediatric oral suspension formulations of amoxicillin/clavulanic acid provides approximately 3 times the clavulanate dose (in milligrams per kilogram per day) than what is currently approved for use in adults.2
Kaizen aims to use this PK/PD modeling and simulation data along with the results from its pharmacokinetic trial to support its NDA application for a novel formulation (amoxicillin and reduced clavulanate) to treat children 3 months and older with infections caused by bacteria.
1Hoberman A, Paradise JL, Rockette HE, Jeong J-H, Kearney DH, Bhatnagar S, Shope TR, Muñiz G, Martin JM, Kurs-Lasky M, Haralam M, Pope MA, Nagg JP, Zhao W, Miah MK, Beumer J, Venkataramanan R, Shaikh N. 2017. Reduced-concentration clavulanate for young children with acute otitis media. Antimicrob Agents Chemother 61:e00238-17. https://doi .org/10.1128/AAC.00238-17.
2Dagan R, Hoberman A, Johnson C, Leibovitz EL, Arguedas A, Rose FV, Wynne BR, Jacobs MR. 2001. Bacteriologic and clinical efficacy of high dose amoxicillin/clavulanate in children with acute otitis media. Pediatr Infect Dis J 20:829–837. https://doi.org/10.1097/00006454-200109000-00002.