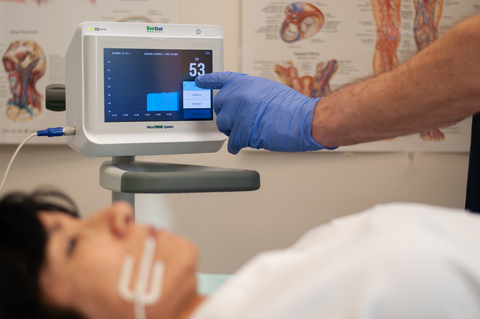
PRIOR LAKE, Minn.--(BUSINESS WIRE)--ExoStat Medical (“ExoStat”), a privately-held medical device company, announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market its MicroTREND System. The MicroTREND was designed to directly detect and monitor tissue hypoperfusion at the microcirculatory hemodynamic level (oral mucosa) as it manifests into a dangerous medical emergency. The MicroTREND incorporates an electro-conductance platform with a disposable sensor that is seated non-invasively in the oral mucosal tissue of a patient, a convenient and accessible site from which to measure pCO2 , a clinically proven marker for tissue hypoperfusion.
ExoStat has been engaged in the research and development of the MicroTREND since 2008. The first clinical work began in the late 1990’s at the Weil Institute of Critical Care Medicine (WICCM) under the guidance of Dr. Max Harry Weil, now known as The Father of Critical Care Medicine. He and his team focused on the capture and measurement of microcirculatory perfusion data using oral tissue as an available and reliable site to measure gut perfusion. Dr. Weil authored many studies and papers on the importance of using oral tissue perfusion pCO2 data to identify early symptoms of microcirculatory hypoperfusion. The MicroTREND is the culmination of decades of research and development. It is the world’s first and only real-time, bedside, non-invasive disposable sensor system approved for identifying and monitoring microcirculatory tissue hypoperfusion resulting from low blood flow.
“Many of today’s top professors, researchers, and clinicians received early training at WICCM under Dr. Weil,” stated Jim Hays, Chief Executive Officer of ExoStat. “As a result of their keen understanding of the need to know a patient’s microcirculatory status, several of them have advised and supported our efforts to create the MicroTREND. These key opinion leaders will lead the early adoption efforts needed to make pCO2 monitoring a standard of care. The 510(k) clearance of the MicroTREND marks an important commercial milestone for ExoStat, and we look forward to working with key strategic partners to advance the next steps of the journey to improve patient care.”
“One area of particular importance to care providers is septic shock,” said Wanchun Tang MD, MCCM, FAHA, FNAI, professor and long-time President at WICCM and Senior Medical and Technical Advisor at ExoStat Medical. “This voracious killer is very complex and difficult to diagnose solely with existing macrohemodynamic parameters. Knowing microcirculatory tissue perfusion status early in septic shock’s predictable cascade will provide physicians with evidence-based data that could lead to better patient outcomes. Monitoring pCO2 data later in the resuscitative process will also aide the care providers in determining the most effective end-point of resuscitation which is also critical to better outcomes for gravely ill ICU patients.”
About ExoStat Medical
ExoStat Medical Inc. is a privately-held medical device company located in Prior Lake, MN. The research and development quest to create the MicroTrend began in 2008 as a vision of Max Harry Weil, MD, who is often called “the father of critical care medicine”. The technology platform developed by ExoStat provides data to enable physicians to assess and to treat microcirculatory hypoperfusion, a life-threatening medical emergency. Visit www.exostatmedical.com for more information.