BARCELONA, Spain--(BUSINESS WIRE)--INBRAIN Neuroelectronics, a brain-computer interface therapeutics (BCI-Tx) company developing graphene-based neural technologies, today announced the close of a $50 million Series B financing round. The round was led by imec.xpand with new investors EIC Fund, Fond ICO Next Tech, CDTI-Innvierte and Avançsa. Existing investors Asabys Partners, Aliath Bioventures and Vsquared also participated, bringing the total amount raised since inception to $68 million.
In addition to the Series B round, INBRAIN also secured additional funding and support from Merck KGaA to advance the clinical development of its technology in Merck’s therapeutic areas of interest. This partnership will boost the translation of INBRAIN’s platform to human use, expanding its impact across both central and peripheral nervous system applications.
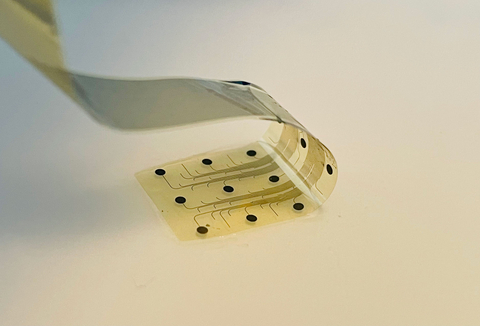
The company’s technology utilizes the unique properties of graphene, a Nobel Prize-winning material known for its strength, flexibility, and conductivity. INBRAIN’s implant is only 10 micrometers thick—thinner than a human hair—and is designed to safely decode and modulate neural signals with exceptional accuracy, offering a new level of performance in the emerging field of precision neurology.
The funding will enable INBRAIN to accelerate the development of its graphene-based BCI-Tx platform, which offers a bidirectional solution to decode and modulate neural activity with unprecedented resolution. The funding will support ongoing clinical trials, enable team expansion and further develop the company’s AI-powered platform for the treatment of neurological-related diseases.
“We’re shaping the future of brain-computer interface therapeutics,” said Carolina Aguilar, CEO and Co-Founder of INBRAIN Neuroelectronics. “This funding empowers us to advance our AI-driven graphene neurotechnology, which has already shown great results versus current commercial neuromodulation technology. We are excited to have the support of a top-tier syndicate as we work to bring this technology to patients in need of more precise, personalized treatments.”
As part of the funding round, INBRAIN is also announcing a collaboration agreement with imec, the world's leading independent nanoelectronics hub. Imec will support INBRAIN in preparing to scale graphene interfaces at a commercial level.
“INBRAIN Neuroelectronics is pioneering a groundbreaking approach to BCI technology using graphene,” said Frank Bulens, Partner at imec.xpand. “This unique graphene-based BCI platform has the potential to redefine how we treat neurological disorders by offering more precise, adaptable, and intelligent therapeutics. We look forward to supporting the INBRAIN team in accelerating their clinical trials and bringing this next-generation technology to patients.”
INBRAIN recently achieved a major milestone with the world’s first human application of its graphene-based BCI in an ongoing clinical trial at Salford Royal Hospital in Manchester, UK. The trial aims to evaluate the safety of INBRAIN’s device in patients with brain cancer and will enroll up to ten participants to assess the superiority of graphene over traditional materials for neural applications.
“The success of INBRAIN’s first patient procedure and recent funding highlights the innovation we envisioned when we launched the Graphene Flagship project,” said Marcin Nowak, Investment Director at European Investment Bank. “By harnessing the remarkable properties of graphene, INBRAIN is advancing cutting-edge neurotechnology applications that could significantly improve patient outcomes and also position Europe as one of the leaders in the global BCI industry. With this important investment, we are proud to support INBRAIN in translating this technology from research into real-world impact on patients' lives.”
About INBRAIN Neuroelectronics
INBRAIN Neuroelectronics is pioneering real-time precision neurology with the world’s first graphene-based brain-computer interface therapeutics (BCI-Tx) platform. Our technology combines advanced neural decoding and micrometric modulation to deliver personalized, adaptive treatments for conditions like Parkinson’s, epilepsy, and stroke rehabilitation. By continuously monitoring and adjusting therapies in real-time, our AI-driven platform enhances outcomes while reducing side effects. This pioneering and disruptive development has led to a FDA Breakthrough Device Designation for INBRAIN’s BCI-Tx in Parkinson’s Disease. In collaboration with partners like Merck KGaA and our subsidiary INNERVIA Bioelectronics, we are expanding these innovations to treat peripheral nerve and systemic diseases, driving the future of neurotechnology and bioelectronics. Visit us at www.inbrain-neuroelectronics.com.