ALAMEDA, Calif.--(BUSINESS WIRE)--Science Corporation, a leader in brain-computer interface (“BCI”) technology, has announced the preliminary clinical trials results for its PRIMA retina implant. The results showed that the implant restored real "form vision" in patients who had lost their central visual field, allowing them to execute useful, high acuity tasks like reading or recognizing faces. After receiving a PRIMA implant, patients were able to read with a clinically meaningful improvement including the ability to recognize letters.
“The results demonstrate a milestone in the treatment of severe vision loss caused by geographic atrophy due to age-related macular degeneration. For the first time it was possible to restore real form vision in a retina that has deteriorated due to age-related macular degeneration,” said Professor Frank Holz, MD, Scientific Coordinator of the PRIMAvera study. “Prior to this, there have been no real treatment options to improve vision for these patients,” added Professor Holz, who is Chairman and Professor, Department of Ophthalmology, at the University of Bonn, Germany.
Max Hodak, Science Corp CEO, added: “To my knowledge, this is the first time that restoration of the ability to fluently read has ever been definitively shown in blind patients. This represents an enormous turning point for the field, and we’re incredibly excited to bring this important technology to market over the next few years.”
The study was designed to demonstrate PRIMA’s safety and efficacy to reach European market approval (CE mark). PRIMA, owned and manufactured by Science Corp, is based on original research done at Stanford University and later by Pixium Vision, which was acquired by Science Corp.
Clinical Trial of 38 Patients
The PRIMA clinical trial, called PRIMAvera, is a clinical study with 38 patients suffering from geographic atrophy (GA), who were implanted with the PRIMA retinal system to restore vision. Geographic atrophy is an advanced form of age-related macular degeneration (AMD) where the central area of the retina, and thus the patient’s sight, has deteriorated.
A Meaningful Improvement of Visual Acuity
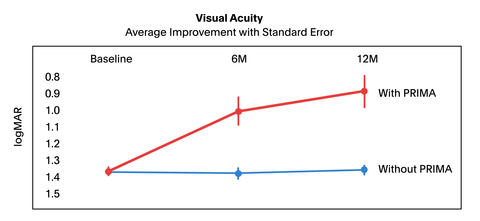
The results show a meaningful improvement of visual acuity when using the system. Patients show a notable improvement in their letter acuity while using the PRIMA implant to read a series of letters; some are able to read longer text.
Preliminary Results
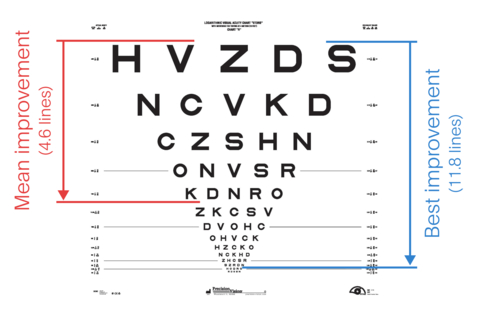
The visual acuity of all participating patients was measured at 6 and 12 months post implantation via logMAR scoring (zero logMAR indicates normal vision, positive values indicate poor vision), and it demonstrated clinically significant improvement:
- A 23 letter (4.6 lines) mean improvement at 12 months post implantation compared to baseline.
- A 59 letter (11.8 line) maximum improvement in the patient with the best response.
- A success was defined as 10 letters (2 lines) of improvement.
- The mean natural visual acuity without using PRIMA remained stable after implantation, which suggests a good safety profile.
- Additional details from the trials can be found here: link.
The PRIMA System
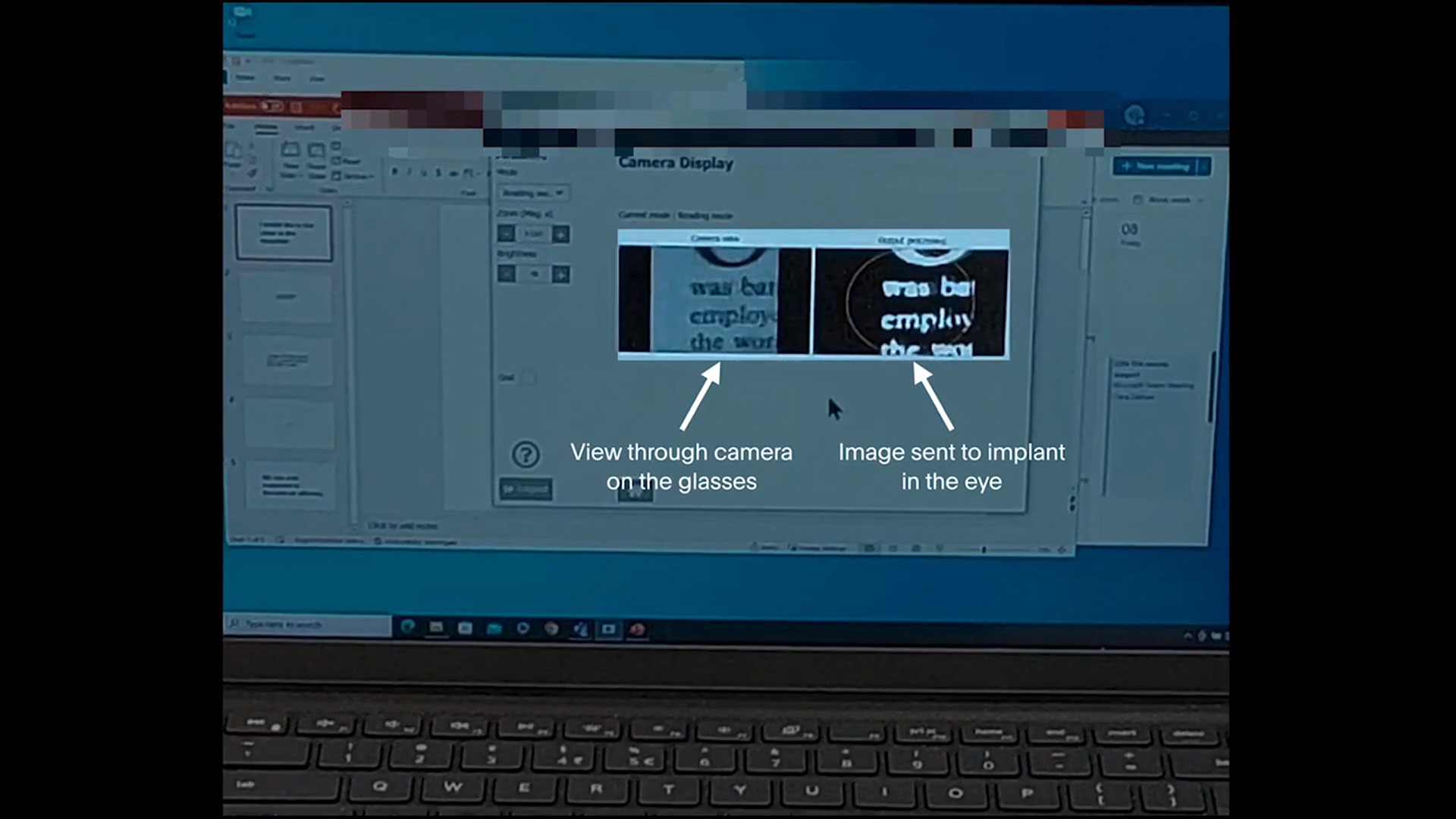
The PRIMA system is a visual prosthesis which consists of a photovoltaic implant—totally cableless and autonomous—surgically implanted under the retina, a special pair of glasses with a camera and built-in projection system, and a pocket processor that processes the image for clarity and magnification.
Additional Background
According to the United States National Institute of Health, there are about one million cases of GA in the US, and about 160,000 new cases occur per year. The American Academy of Ophthalmology estimates that over eight million people are affected worldwide with GA. The most significant risk factors are increasing age and family history.
About Science Corporation:
Science Corporation is a leader in the field of brain-computer interface (BCI) technology, and the emerging field of neural engineering broadly. We work to restore quality of life to those with debilitating conditions for which there are no treatment options, creating devices aimed at restoring vision, cognition, and mobility. To support progress across our industry, we provide state-of-the-art components and vertically integrated infrastructure for others to build on via Science Foundry. We are headquartered in Alameda, California with additional offices in Research Triangle Park, North Carolina and Paris, France.