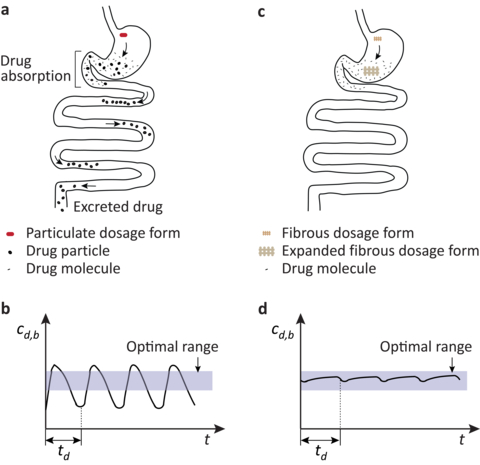
ZURICH & CAMBRIDGE, Mass.--(BUSINESS WIRE)--At present, the prevalent oral solid dosage forms, the capsules and tablets, are loose or lightly compacted mixtures of drug and excipient particles. Upon ingestion, they rapidly disintegrate into their constituents in the stomach. The small drug particles then are swept out of the stomach and pass through the intestine.
Many kinds of cancer drug, however, are absorbed only in the acidic upper part of the gastrointestinal tract. Because the gastric residence time of the drug particles (~2-3 hours) is much shorter than the convenient dosing interval (~12-24 hours), upon repeated dosing of such drugs the drug concentration in blood fluctuates greatly.
This is therapeutically not optimal: The maximum drug concentration is high, promoting such acute side effects as heart problems, high blood pressure, liver damage, headache, and so on, and the minimum is low, compromising the efficacy of the therapy.
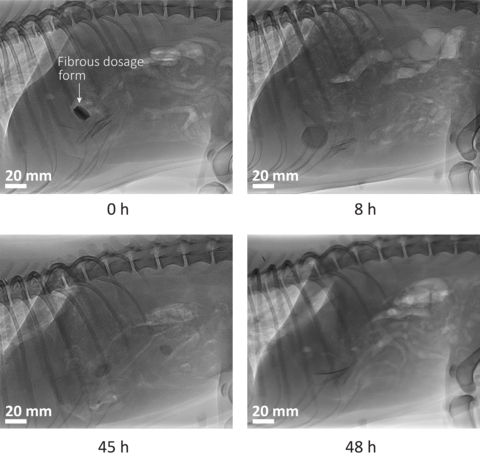
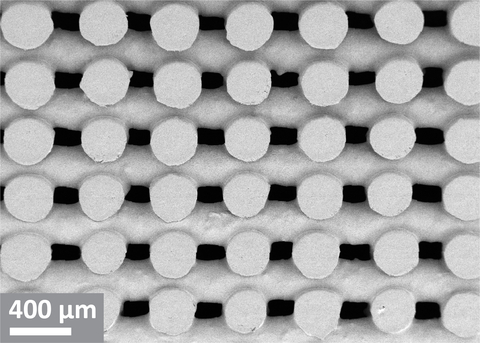
To mitigate these limitations, Enzian Pharmaceutics has invented, and developed, a new dosage form comprising a cross-ply structure of cellulose fibers. Upon ingestion, the fibrous dosage form expands due to water absorption, and forms an expanded viscoelastic gel that resides in the stomach for prolonged time, thereby releasing drug at a constant rate.
The concept was validated on animals in a two-part study [1,2] conducted at the Diagnostic Imaging Research Unit (DIRU), University of Zurich, and published in the International Journal of Pharmaceutics (IJP). It was shown that the Enzian dosage form resides in the stomach of dogs and pigs for about a day, and dissolves for safe excretion thereafter. In a follow-up four-part study [3,4,5,6], also published by IJP, it was shown that the dosage form delivers cancer drugs into the blood of dogs at a constant rate, ensuring a steady drug concentration in blood over time.
Thus, the Enzian dosage forms enable maintaining the optimal drug concentration in blood, thereby enhancing efficacy and mitigating unacceptable side effects of cancer therapies. According to Dr. Aron Blaesi, Enzian’s founder, CEO, and Lead Scientist, work is currently underway to validate the dosage forms in humans.
References:
- https://doi.org/10.1016/j.ijpharm.2021.120792
- https://doi.org/10.1016/j.ijpharm.2022.122378
- https://doi.org/10.1016/j.ijpharm.2024.124360
- https://doi.org/10.1016/j.ijpharm.2024.124361
- https://doi.org/10.1016/j.ijpharm.2024.124362
- https://doi.org/10.1016/j.ijpharm.2024.124363