CRYSTAL LAKE, Ill.--(BUSINESS WIRE)--Aptar Digital Health, a global leader in digital health solutions including Software as a Medical Device (SaMD), digital Patient Support Programs, connected devices, and disease management platforms, today announced a collaboration with Guy’s and St Thomas’ NHS Foundation Trust, a leading Trust in London, United Kingdom, to evaluate the impact of Aptar Digital Health’s Respiratory Disease Management Platform, ADH Respiratory DMP, on asthma patients. Both organizations are partnering with the Clinical Research Organization (CRO) Lindus Health to execute the study.
The clinical study, which began in July 2024 and will run for twelve months, will recruit 118 adults with moderate to severe asthma, with the primary aim of assessing the improvement of asthma symptom control.
During the study, clinical data, including asthma control symptoms, quality of life, asthma controller medication adherence, healthcare resource utilization and overall satisfaction with the digital solution, will be evaluated.
About Aptar Digital Health’s ADH Respiratory DMP
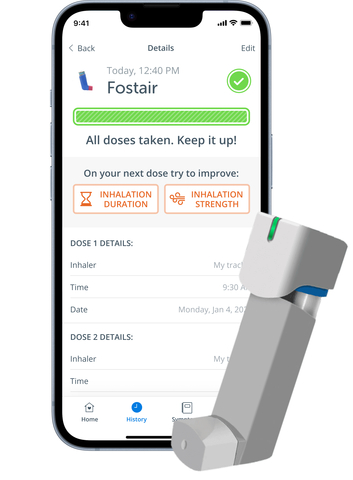
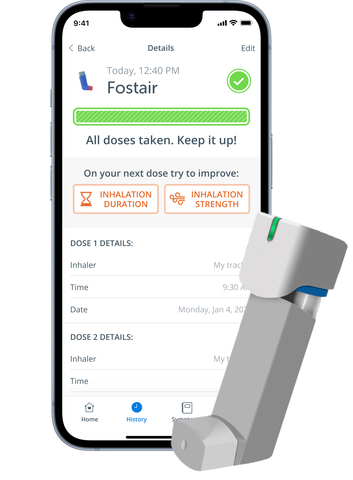
The ADH Respiratory DMP is designed to improve asthma symptom control, technique and engagement. It includes a patient mobile application that connects to inhaler sensors, such as Aptar Digital Health’s HeroTracker® Sense, and a software portal for healthcare professionals. It aims to empower individuals by offering medication and symptoms management with real-time tracking, reminders and educational resources via a mobile app. By fostering behavior change and facilitating adherence, this digital platform aims to reduce asthma symptoms, rescue medication usage, exacerbations and unnecessary healthcare visits.
The United Kingdom has a high prevalence of asthma, with over 12% of its population1 suffering from the disease. An asthma attack occurs approximately every eight minutes, leading to hospitalization, resulting in more than 60,000 hospital admissions and 200,000 hospital bed days every year. The National Health Service (NHS) allocates about £1 billion annually to treat and manage asthma. Moreover, 65% of asthma patients do not receive fundamental care from healthcare providers, such as annual health checks, proper inhaler usage checks, and a personalized treatment plan. Almost 40% of inhaler prescriptions are refilled without any consultation with healthcare professionals, underscoring the need for asthmatic patients to be properly educated on correct inhaler techniques to ensure effective medication use and dosage, and reduce the risk of severe and potentially fatal asthma attacks2.
Geneviève d’Orsay, Chief Medical Officer at Aptar Digital Health, said, “Asthma affects 334 million people worldwide3, with between a third and one-half of asthma sufferers having severe symptoms that regularly interfere with everyday life4. Our respiratory platform previously demonstrated potential to improve asthma control and decrease rescue medication use in the U.S.,5 and we are excited to study our enhanced platform as part of this clinical study.”
Added Pierre Leurent, President Patient Services and Digital Health at Aptar Pharma, "Enhancing the patient experience is the mission of Aptar Digital Health and we are delighted to achieve this through our collaboration with Guy's and St Thomas’ NHS Foundation Trust, a leading healthcare organization. Effective management of asthma is necessary to mitigate the consequences of the disease, and we believe that digital solutions can deliver a positive impact on the lives of people affected.”
Professor David Jackson, Consultant in Asthma & Eosinophilic Lung Diseases at Guy’s and St Thomas’ NHS Foundation Trust, and Chief Investigator of the clinical study, commented, “Poor adherence to inhaled steroids remains the biggest barrier to asthma control. At the same time, a failure to identify and correct suboptimal adherence leads to unnecessary escalation to very costly biologic agents due to the incorrect assumption many patients have severe asthma when they do not. As such, electronic monitoring of ICS adherence has enormous potential to both improve quality of care and reduce healthcare costs in asthma, and we are excited to start this study with the aim of demonstrating this.”
About Aptar Digital Health
Aptar Pharma's Digital Health division is part of AptarGroup, Inc., a global leader in drug and consumer product dosing, dispensing and protection technologies. Aptar Digital Health creates end-to-end solutions to enhance patient experiences every day, leveraging a holistic ecosystem of digital interventions. Amplified by an industry-leading portfolio of products and solutions, Aptar Digital Health’s offering combines mobile and web apps, connected drug delivery systems, onboarding, training, and advanced data analytics services to actively empower patients and create a positive treatment journey. Aptar is headquartered in Crystal Lake, Illinois and has more than 13,000 dedicated employees in 20 countries. For more information, visit www.aptardigitalhealth.com and www.aptar.com.
| _______________________________ |
1 What is the prevalence of asthma? NICE. (2022, April). Retrieved February 4, 2023, from https://cks.nice.org.uk/topics/asthma/background-information/prevalence/ |
2 Pharmaceutical Services Negotiating Committee (PSNC). Essential facts, stats, and quotes relating to asthma. March 28, |
3 Enilari, O., Sinha, S. (2019). The global impact of asthma in adult populations. Ann Glob Health, 85(1), 2, 1-7. |
5 Bijlani A, Mauger D, Goodheart C, dOrsay G, Suman J. Impact of a digital therapeutic on adult asthma. Eur J Public Health. 2023 Oct 24;33(Suppl 2):ckad160.823. doi: 10.1093/eurpub/ckad160.823. PMCID: PMC10597004 |