BOSTON--(BUSINESS WIRE)--Novotech, the global full-service clinical Contract Research Organization (CRO) that partners with biotech companies to accelerate the development of advanced and novel therapeutics at every phase, has released its latest industry report, Pancreatic Cancer- Global Clinical Trial Landscape (2024).
The landscape of pancreatic cancer (PC) research is experiencing a transformative phase, with significant advancements in clinical trials, drug development, and therapeutic strategies. The 2024 Global Clinical Trial Report offers critical insights into these developments, showcasing the growing global effort to combat one of the most lethal forms of cancer.
Key Highlights:
Global Clinical Trials:
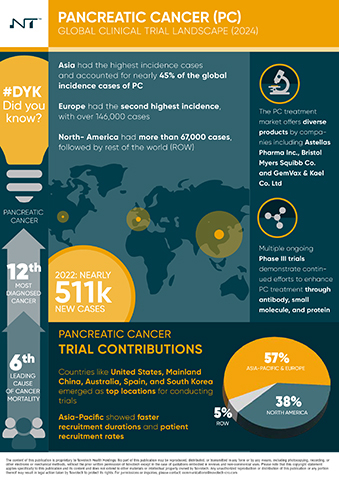
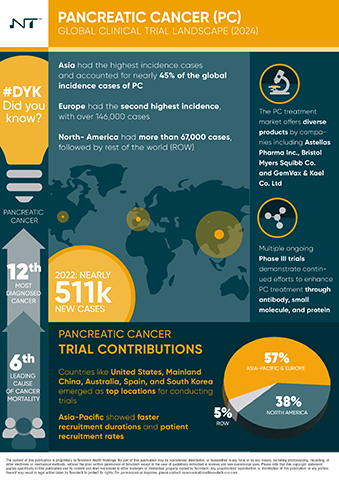
- Over 2,000 PC clinical trials have been initiated worldwide since 2019, with North America and the Asia-Pacific region leading the charge, each contributing 38% and 37% of the trials, respectively. Europe follows with a 20% share.
- The United States leads North America with 88% of the trials, while Mainland China dominates the Asia-Pacific region with 43% of trials. Other key contributors include Australia, South Korea, and Japan.
Drug Development Landscape:
- The report identifies 164 drugs in preclinical stages and 233 in early-phase clinical trials, 29 in Phase III, and 11 already on the market, highlighting a robust and growing pipeline aimed at improving treatment outcomes for PC patients.
- Notably, small molecules remain the primary focus of development, with prominent companies such as Astellas Pharma Inc., Bristol Myers Squibb Co., and Eli Lilly and Co. leading the market with their innovative treatments.
- The drug development landscape also includes significant contributions from Pfizer Inc., Roche, and Merck & Co. Inc., with a strong emphasis on targeted therapies and immunotherapies.
Therapeutic Innovations:
- Recent years have seen advancements in neoadjuvant chemotherapy, RAS-directed therapies, and immunotherapy, offering new hope to PC patients. Stroma-modifying drugs are also showing promise in clinical trials, potentially revolutionizing treatment approaches.
- The report predicts that the future of PC treatment will see a greater focus on personalized medicine, early detection methods, and targeted biological therapies, which are expected to significantly improve survival rates and quality of life for patients.
Regional Insights and Trial Density:
- Despite its large population, the Asia-Pacific region's clinical trial density is six times lower than that of the US and about half that of Europe, signaling an urgent need for increased research and targeted medical interventions in these regions.
- The Asia-Pacific region's untapped research potential, combined with its vast patient population and unique genetic diversities, presents a prime opportunity for groundbreaking clinical research and the development of tailored therapeutic interventions.
Leading Contributors:
- Companies like Astellas Pharma Inc., Bristol Myers Squibb Co., and Eli Lilly and Co. have made significant contributions to the PC drug market. The presence of advanced trials by giants such as Pfizer Inc., Roche, and Merck & Co. Inc. underscores the collective global effort to address the challenges posed by pancreatic cancer.
Strategic Insights:
- The SWOT analysis of the pancreatic cancer treatment landscape reveals significant strengths in the development of targeted therapies and immunotherapies, bolstered by increased research funding. However, challenges such as late diagnosis, low survival rates, and the lack of early detection biomarkers persist.
- Opportunities lie in personalized medicine and growing research investments, while threats include stringent regulatory hurdles and the prohibitive cost of advanced treatments, potentially limiting patient access.
The report underscores the critical importance of continued investment in PC research and development. With the backing of leading pharmaceutical companies and innovative clinical trials, the future of pancreatic cancer treatment is bright, offering new hope to patients and their families.
For more information and to download the full report free of charge, please visit Novotech's website or contact our media relations team.
About Novotech Novotech-CRO.com
Founded in 1997, Novotech is a global full-service clinical Contract Research Organization (CRO) focused on partnering with biotech companies to accelerate the development of advanced and novel therapeutics at every phase.
Recognized for its industry-leading contributions, Novotech has received numerous prestigious awards, including the CRO Leadership Award 2023, the Asia Pacific Cell & Gene Therapy Clinical Trials Excellence 2023, the Asia-Pacific Contract Research Organization Company of the Year Award since 2006.
The Company offers a comprehensive suite of services including laboratories, Phase I facilities, drug development consulting, regulatory expertise, and has experience with over 5,000 clinical projects, including Phase I to Phase IV clinical trials and bioequivalence studies. With a presence in 34 office locations and a dedicated team of 3,000+ professionals worldwide, Novotech is a trusted end-to-end strategic partner of choice.
For more information or to speak to an expert team member visit www.Novotech-CRO.com