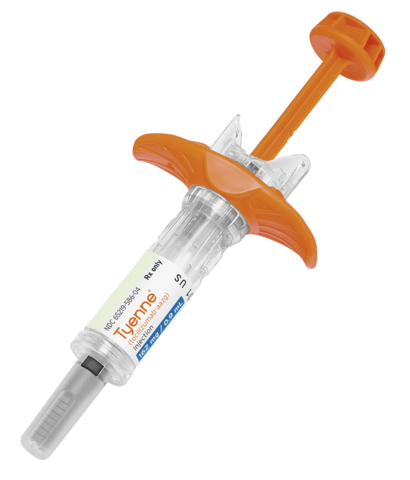
LAKE ZURICH, Ill.--(BUSINESS WIRE)--Fresenius, via its operating company Fresenius Kabi, announced today the immediate availability in the U.S. of its biosimilar Tyenne® (tocilizumab-aazg), in a subcutaneous formulation, which will increase access to affordable and cost-effective treatment options for use in the treatment of chronic autoimmune diseases.
Tyenne is indicated for use in the treatment of chronic autoimmune diseases for certain indications of the reference product, Actemra® (tocilizumab). Fresenius Kabi previously launched Tyenne® in an intravenous (IV) formulation in April of this year.
“After successfully launching Tyenne in more than 10 countries, we are on track to continue the momentum in the U.S with the launch of a second formulation,” said Sang-Jin Pak, M.D., President of Biopharma at Fresenius Kabi. “Introducing our subcutaneous formulation demonstrates our continued commitment to providing greater access to more patients living with autoimmune diseases.”
Trial data that compared Tyenne® to the reference product, Actemra® showed similar safety and tolerability.
Tyenne® is the first tocilizumab biosimilar with an intravenous and subcutaneous formulation approved by the FDA. Tyenne® received FDA approval on March 5, 2024, and an IV formulation was launched in April 2024 for use in the treatment of several inflammatory autoimmune diseases, including rheumatoid arthritis, giant cell arteritis, polyarticular juvenile idiopathic arthritis, and systemic juvenile idiopathic arthritis.
To learn more about how Fresenius Kabi provides comprehensive patient support for Tyenne® in the U.S. please click here.
About Tyenne®, a Tocilizumab Biosimilar
Tyenne® (tocilizumab-aazg), a biosimilar to Actemra® (tocilizumab), is a prescription medicine called an Interleukin-6 (IL-6) receptor antagonist. It was developed by Fresenius Kabi using advanced analytical and manufacturing technologies for use in the treatment of several autoimmune diseases, including rheumatoid arthritis, giant cell arteritis, polyarticular juvenile idiopathic arthritis, and systemic juvenile idiopathic arthritis. Tyenne® includes a Boxed Warning for serious infections leading to hospitalization or death including tuberculosis (TB), bacterial, invasive fungal, viral, and other opportunistic infections that have occurred in patients receiving tocilizumab products. Tyenne® is contraindicated in patients with known hypersensitivity to tocilizumab products. For more information about Tyenne®, please see the important safety information and full prescribing information for the U.S.
About Fresenius Kabi
Fresenius Kabi is a global healthcare company that specializes in lifesaving medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used for the therapy and care of critically and chronically ill patients.
Its product portfolio comprises a range of highly complex biopharmaceuticals, clinical nutrition, medical technologies, and I.V. generic drugs. Within biopharmaceuticals, Fresenius Kabi offers, among others, biosimilar drugs with a focus on autoimmune diseases and oncology. The company’s clinical nutrition offering includes a wide selection of enteral and parenteral nutrition products. In the segment of medical technologies, its offering includes vital disposables, infusions pumps, apheresis machines, cell therapy devices, and more. Fresenius Kabi puts essential medicines and technologies in the hands of people who help patients and finds the best answers to the challenges they face.
Following its strategy “Vision 2026”, which is a key part of the #FutureFresenius program of the Fresenius healthcare group, the company is furthermore committed to increase efficiencies in the therapy and care of patients and improve access to high-quality healthcare around the globe. Fresenius Kabi aspires to be leader globally in its product segments – all for the benefit of patients, its customers, and its stakeholders.
Fresenius SE & Co. KGaA (Frankfurt/Xetra: FRE) is a global healthcare company headquartered in Bad Homburg v. d. Höhe, Germany. In the 2023 fiscal year, Fresenius generated €22.3 billion in annual revenue with its more than 190,000 employees. Fresenius offers solutions to the social challenges posed by a growing and ageing population and the resulting need for affordable, high-quality healthcare. The Fresenius Group comprises the operating companies Fresenius Kabi and Fresenius Helios as well as the investment companies Fresenius Vamed and Fresenius Medical Care. With 140 hospitals and countless outpatient facilities, Fresenius Helios is the leading private hospital operator in Germany and Spain, treating around 26 million patients every year. Fresenius Kabi’s product portfolio
includes a range of highly complex biopharmaceuticals, clinical nutrition, medical technology, and generic intravenous drugs. Fresenius was established in 1912 by the Frankfurt pharmacist Dr. Eduard Fresenius. After his death, Else Kröner took over management of the company in 1952. She laid the foundations for a global enterprise that today pursues the goal of improving people’s health. The largest shareholder is the non-profit Else Kröner-Fresenius Foundation, which is dedicated to advancing medical research and supporting humanitarian projects.
For more information visit the company website at www.fresenius.com.
Follow us on social media: www.fresenius.com/socialmedia.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g., changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius SE & Co. KGaA
Registered Office: Bad Homburg, Germany / Commercial Register: Amtsgericht Bad Homburg, HRB 11852
Chairman of the Supervisory Board: Wolfgang Kirsch
General Partner: Fresenius Management SE
Registered Office: Bad Homburg, Germany / Commercial Register: Amtsgericht Bad Homburg, HRB 11673
Management Board: Michael Sen (Chairman), Pierluigi Antonelli, Sara Hennicken, Robert Möller, Dr. Michael Moser
Chairman of the Supervisory Board: Wolfgang Kirsch
Actemra® is a registered trademark of Chugai Seiyaku Kabushiki Kaisha Corp., a member of the Roche Group.
Tyenne® is a registered trademark of Fresenius Kabi Deutschland GmbH.