COPENHAGEN--(BUSINESS WIRE)--In Q1 2024, LEO Pharma delivered a revenue growth of 13% in constant exchange rates (CER). The dermatology portfolio saw accelerated growth in revenue of 16%. The acquisition of TMB-001 to the treatment of congenital ichthyosis added a late-stage asset to LEO Pharma’s medical dermatology pipeline, and delgocitinib for chronic hand eczema (CHE) is on track for its planned European launch in Q4 2024. Full-year outlook has been revised slightly upwards.
Q1 2024 financial highlights
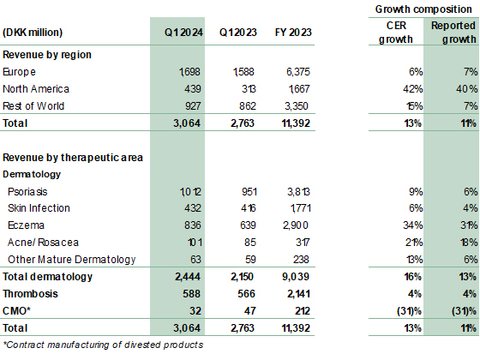
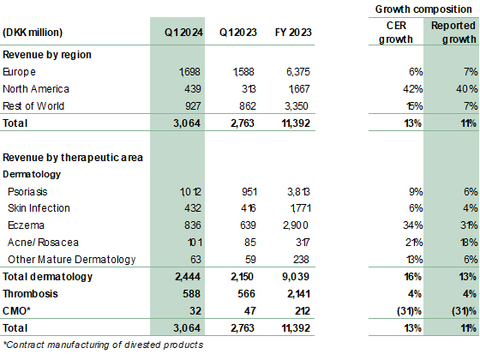
- Revenue grew 13% to DKK 3,064 million (Q1 2023: 2,763 million). Reported growth was 11%.
- Dermatology revenue grew 16% to DKK 2,444 million (Q1 2023: 2,150 million), driven by growth of Adtralza®/Adbry® for atopic dermatitis (AD) and solid growth in the core portfolio.
- Adbry®/Adtralza® revenue increased by 90% driven by continued uptake across markets.
- Core dermatology portfolio delivered growth of 9% across our Affiliate and Alliance markets. Growth largely driven by the Fucidin® Range, Enstilar® and Protopic® across our eczema, psoriasis and skin infection portfolios.
- Growth across all regions: North America up 42%, Europe up 6%, Rest of the World up 15%. North America continues to be the key growth driver with revenue of DKK 439 million (Q1 2023: 313 million).
Progress on strategic priorities
- To advance the growth of our U.S. business, LEO Pharma added a late-stage asset to our medical dermatology pipeline by finalizing the strategic acquisition of TMB-001 to the treatment of congenital ichthyosis. In Q1 2024, patient recruitment was completed for the Phase III ASCEND trial.
- The recent results of the successful DELTA FORCE-trial comparing delgocitinib to current standard of care, further confirms delgocitinib’s potential to become a key growth driver for the company and we will be investing additional resources in accelerating the launch of this potential treatment to patients.
- Regulatory review of the marketing authorization application is pending in the EU, and based on the positive outcome of the DELTA clinical program, LEO Pharma is assessing ways to bring this potential treatment to other markets as well, including the U.S.
- Adbry® (tralokinumab-ldrm) data from the ECZTEND trial was shared during the American Academy of Dermatology (AAD) Annual Meeting investigating the stability of long-term therapeutic responses to tralokinumab in adults with moderate-to-severe AD.
“First quarter marks a solid start to 2024 with a 13% revenue growth and continued progress on our strategic priorities. I am pleased with the strong growth track record for our global dermatology portfolio, primarily driven by performance in North America. Furthermore, I am excited about the progress we are making in building evidence and bringing delgocitinib to the market to help patients with chronic hand eczema. The positive results from the recently completed head-to-head trial against current standard of care make us even more motivated to accelerate our efforts,” says CEO Christophe Bourdon.
2024 financial outlook
- Following our strong Q1 2024 performance, we are revising our full-year revenue growth outlook upwards compared to what was shared in the Annual Report on 29th February 2024. Full-year revenue growth is now projected to be 5-8% in CER (before: 4-8%).
- Potential changes in key assumptions for market growth and unexpected health care and pricing reforms are risk factors, among others, which could change the outlook for the year.
Sales performance
In Q1 2024, revenue grew 13% (CER) to DKK 3,064 million from DKK 2,763 million in the same period last year, corresponding to a reported growth in DKK of 11%. Dermatology revenue was a significant contributor with 16% in CER. While growth is attributed to the performance of both recently launched dermatology products Adtralza®/Adbry®, as well as core dermatology products, a part of growth was also attributable to timing of shipments especially in rest of world.
LEO Pharma expects to issue a company announcement on the financial performance and progress on strategic priorities in the first half of 2024 on 26 August 2024.
Ballerup, 3 May 2024,
LEO Pharma
About LEO Pharma
LEO Pharma is a global company dedicated to advancing the standard of care for the benefit of people with skin conditions, their families and society. Founded in 1908 and majority owned by the LEO Foundation, LEO Pharma has devoted decades of research and development to advance the science of dermatology, and today, the company offers a wide range of therapies for all disease severities. LEO Pharma is headquartered in Denmark with a global team of approx. 4,200 people, serving millions of patients across the world. In 2023, the company generated net sales of DKK 11.4 billion.
Forward-looking statements
This announcement contains forward-looking statements, including forecasts of future revenue and operating profit, as well as expected business-related events. Such statements are subject to risks and uncertainties, as various factors, some of which are beyond LEO Pharma’s control, may cause actual results and performance to differ materially from the forecasts made in this announcement.