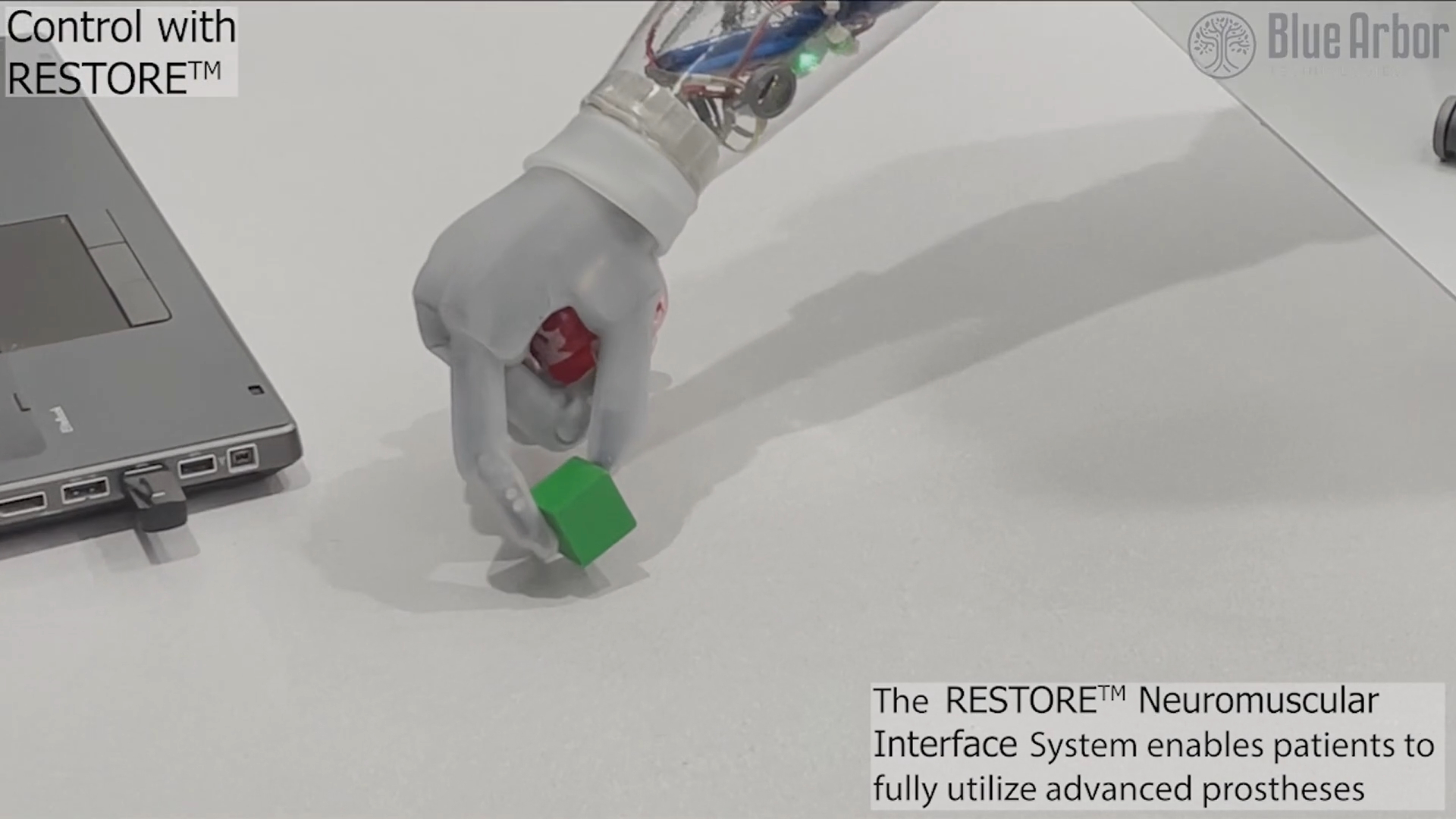
GRASS LAKE, Mich.--(BUSINESS WIRE)--Blue Arbor Technologies Inc., a company developing novel neuromuscular interfaces and next-generation robotic prosthetic control systems for people with limb loss, announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to the RESTORE™ Neuromuscular Interface System for people with upper limb loss. The system has also been accepted into the FDA’s Total Product Life Cycle (TPLC) Advisory Program (TAP) Pilot. The RESTORE System is designed to seamlessly integrate the peripheral nervous system with commercially available robotic prosthetics to restore naturalistic hand and arm function in patients with upper limb loss. The platform may enable patients to move their upper limb prosthetic devices with unprecedented dexterity, speed, and reliability.
“For decades, researchers and physicians have been trying to solve the problem of limited connectivity between patients and their protheses, so that they can function intuitively just like human limbs,” said Aviram Giladi, M.D., research director at The Curtis National Hand Center in Baltimore, MD., and a pioneer in limb reconstruction. "Early studies show the RESTORE Neuromuscular Interface System has the potential to be transformative for people with prosthetic upper limbs, providing hope for more controlled, seamless, and natural functioning. It could be like they’re getting a hand back.”
Unlike current interface technologies that are dependent on surface skin electrodes, the RESTORE System is designed to provide a direct connection to the patient’s residual muscles and peripheral nerves to deliver reliable, voluntary movement control signals. This direct connection provides unparalleled control of currently available advanced robotic prostheses. In addition, the RESTORE System is designed to allow for simultaneous and independent movement of finger, wrist, and elbow joints. This level of capability far exceeds what is possible with surface skin electrodes. Furthermore, the RESTORE System has been shown to consistently capture motor signals for over five years in early feasibility human trials.1
Approximately one in 200 Americans is living with limb loss2 and it is projected that this number could double by 2050.3 Upper limb loss affects an estimated 595,000 people in the United States, with another 65,000 additional people sustaining limb loss each year.4 A 2022 study found that despite recent advancements in technology, more than 44% of people with upper limb loss abandoned their prostheses, citing problems with discomfort, heaviness, and functionality.5
“Blue Arbor Technologies is dedicated to developing solutions that address the unmet need for improved robotic prosthetic control options for people with limb loss so they can more easily integrate a prothesis in their daily lives and return to normality,” said Paul Cederna, M.D., president of Blue Arbor Technologies. “The Breakthrough Designation and TAP enrollment is a valuable step in the pathway to FDA market clearance. We look forward to working closely with the agency to make the RESTORE System available to people with upper limb loss to hopefully increase prosthesis adoption and use.”
The FDA Breakthrough Devices Program expedites the development, assessment, and review process for medical devices that provide more effective treatment options for patients with life-threatening or irreversibly debilitating diseases or conditions. It is designed to ensure that patients and healthcare providers have more timely access to these novel, new medical devices. The TAP Pilot is intended to help streamline collaboration between the FDA and medical device sponsors to accelerate the development and path to commercialization of innovative devices in the breakthrough program.
About the RESTORE Neuromuscular Interface System
The RESTORE System is an investigational neuroprosthetic interface designed to detect voluntary nerve and muscle control signals to facilitate naturalistic function of upper limb prosthetics. The system consists of: 1) Implantable intramuscular electrodes; 2) A sensing unit that filters, conditions, and processes patient-generated electromyographic (EMG) signals and wirelessly transmits these signals to a socket-mounted receiver; 3) A socket-mounted receiver that decodes these signals into movement commands for the prosthesis; and 4) A software package that controls the entire system. It is designed to be compatible with any commercially available robotic upper extremity prothesis. The system can be implanted at the time of an amputation or at the time of any upper limb reconstructive operation for the treatment of neuroma pain and phantom limb pain or for revising the shape of a residual limb.
For more information about the RESTORE System and Blue Arbor Technologies please visit https://bluearbortech.com.
About Blue Arbor Technologies
Blue Arbor Technologies is a medical device company devoted to the development of next-generation robotic control systems for people with limb loss so they can intuitively, accurately, and reliably control a prosthetic device with unparalleled degrees of freedom. The Restore™ System is a neuromuscular interface system that provides a direct connection between the patient’s peripheral nervous system and their robotic upper extremity prosthetic limb. Blue Arbor Technologies is a privately held company located in Grass Lake, Michigan. For more information, please visit https://bluearbortech.com and connect on LinkedIn, Instagram, YouTube, and Facebook.
1 Tian, Y., et al Merging Humans and Neuroprosthetics through Regenerative Peripheral Nerve Interfaces. Semin Plast Surg. 2024 Feb 6;38(1):10-18. DOI: https://pubmed.ncbi.nlm.nih.gov/38495064/
2 Adams Patricia F., et al, Current Estimates from the National Health Interview Survey. Vital and Health Statistics 1996; 1999: 10.(200)
3 Ziegler-Graham K, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008 Mar;89(3):422-9. DOI:https://pubmed.ncbi.nlm.nih.gov/18295618/
4 Ziegler-Graham K, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008 Mar;89(3):422-9. DOI:https://pubmed.ncbi.nlm.nih.gov/18295618/
5 S Salminge, et al. Current rates of prosthetic usage in upper-limb amputees - have innovations had an impact on device acceptance? Disabil Rehabil. 2022 Jul;44(14):3708-3713. DOI:https://pubmed.ncbi.nlm.nih.gov/33377803/