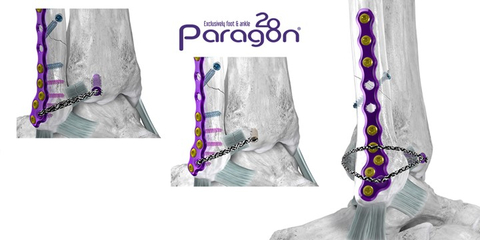
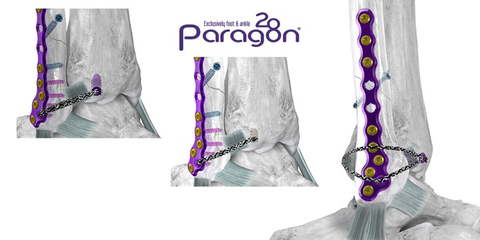
ENGLEWOOD, Colo.--(BUSINESS WIRE)--Paragon 28, Inc. (NYSE: FNA), is pleased to announce the launch of the Grappler® R3INFORCE™ Extraosseous Repair System designed to restore stability to the anterior and posterior ligaments surrounding the ankle during fibula fracture repairs and high ankle sprains.
Upon determination of syndesmotic instability, the Grappler® R3INFORCE™ Anchors are placed in plate holes or other anatomic locations where pre-loaded suture tape can be routed and tensioned to address the injured ligament or ligaments. The Dynamic Anchor allows for micromotion in the repair to better replicate the mechanics of the native tissue.
This system is highly synergistic with Paragon 28’s existing offerings and can utilize the recently launched Grappler® Knotless Sutures. This compatibility allows for multiple configurations for surgeons to cater their syndesmotic repair to their own treatment philosophies.
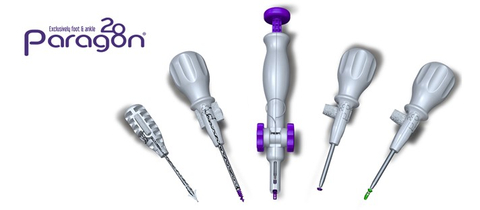
The Grappler® R3INFORCE™ Extraosseous Repair System is sterile packed and includes all instrumentation needed to complete the case in multiple configurations, reducing waste and intraoperative complexity.
Paragon 28’s CEO, Albert DaCosta, commented, “With up to 25% of all ankle fractures as well as unstable high ankle sprains requiring implants to facilitate soft tissue healing, syndesmotic stabilization is a major priority for Paragon 28 and an opportunity for us to improve patients’ recoveries. In launching the Grappler® R3INFORCE™ System and Dynamic Knotless Anchor, we are adding to our comprehensive and growing suite of novel solutions designed specifically to treat injuries to the highly complex syndesmosis.”
Surgeon Designer Mark Davies, MD, commented, “Congratulations to the blue sky thinking and creativity of the Paragon 28 team in manufacturing the Grappler® R3INFORCE™ Extraosseous Repair System. It offers surgeons a comprehensive, physiological, and anatomical reconstruction system for ankle syndesmotic injuries.”
The Grappler® R3INFORCE™ Extraosseous Repair System bolsters Paragon 28’s soft tissue offering, which includes the Paratrooper™ Plantar Plate System, TenoTac™ 2.0 Soft Tissue Fixation System, Grappler® Interference Screw System, Grappler® Suture Anchor System, Grappler® Knotless Anchor System, Bridgeline™ Tape, R3ACT® Stabilization System, R3LEASE™ Stabilization System, and Mister Tendon™ Harvester. With this comprehensive portfolio, Paragon 28® provides its customers with a single source to address their foot and ankle soft tissue needs.
About Paragon 28, Inc.
Based in Englewood, CO., Paragon 28, is a leading medical device company exclusively focused on the foot and ankle orthopedic market and is dedicated to improving patient lives. From the onset, Paragon 28® has provided innovative orthopedic solutions, procedural approaches and instrumentation that cover a wide range of foot and ankle ailments including fracture fixation, forefoot, ankle, progressive collapsing foot deformity (PCFD) or flatfoot, Charcot foot and orthobiologics. The company designs products with both the patient and surgeon in mind, with the goal of improving outcomes, reducing ailment recurrence and complication rates, and making the procedures simpler, consistent, and reproducible.
Forward Looking Statements
Except for the historical information contained herein, the matters set forth in this press release are forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: Paragon 28’s potential to shape a better future for foot and ankle patients. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking statements are only predictions based on our current expectations, estimates, and assumptions, valid only as of the date they are made, and subject to risks and uncertainties, some of which we are not currently aware. Forward‐looking statements should not be read as a guarantee of future performance or results and may not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. These forward‐looking statements are based on Paragon 28’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward‐looking statements as a result of these risks and uncertainties. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Paragon 28’s business in general, see Paragon 28’s current and future reports filed with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and its Quarterly Reports on Form 10-Q, as updated periodically with its other filings with the SEC. These forward-looking statements are made as of the date of this press release, and Paragon 28 assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law.
Disclaimer
Dr. Davies may report consulting and royalty fees from Paragon 28 in connection with the provision of product development services to Paragon 28.
Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations.