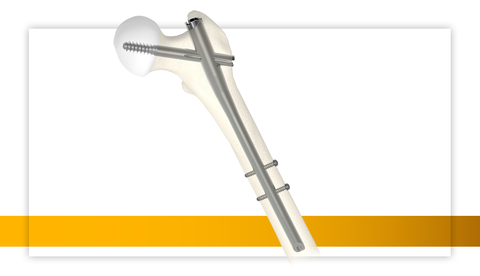
KALAMAZOO, Mich.--(BUSINESS WIRE)--Stryker (NYSE: SYK), one of the world’s leading medical technologies companies, has expanded its Gamma4 Hip Fracture Nailing System, with the addition of an intermediate nail, the RC Lag Screw, and an anti-rotation clip with sleeve components. Since its launch in August of 2022, the Gamma4 System has been used in over 19,000 cases across more than 850 facilities, solidifying its position as a leading choice in orthopaedic procedures.
"The Gamma4 System, with its rich 30-year legacy of innovation and clinical history, has ushered in a new era of intramedullary nailing systems for hip fractures,” said Eric Tamweber, Vice President and General Manager, Stryker’s Trauma business. “Stryker remains unwavering in our commitment to providing cutting-edge orthopaedic solutions to meet the needs of our customers. While Gamma4 has already made a significant impact, the new line extension expands our nailing portfolio and provides surgeons with options that are designed to streamline operative flow and improve procedural efficiency.”
The Gamma4 System, which launched in 2022, is indicated for the treatment of stable and unstable fractures, as well as for stabilization of bones and correction of bone deformities in the intracapsular, trochanteric, subtrochanteric and shaft regions of the femur (including osteoporotic and osteopenic bone). The recent line extension, including the Gamma4 intermediate nail and RC Lag Screw, received 510(k) clearance in June 2023. Key features include:
- Increased cutout resistance: The Gamma4 RC Lag Screw increases cutout resistance by 15% compared to the standard Gamma4 lag screw.1,2
- Increased fixation: The RC lag screw blades allow for 2mm of additional surface area contact within the femoral head compared to the standard Gamma4 lag screw.3
- New intermediate nail option: Designed to provide more isthmus support than a trochanteric nail and faster procedural time compared to a long nail.3,4
- Controlling intraoperative rotation: The anti-rotation clip and sleeve components provide intraoperative stability in rotationally unstable femoral head neck fragments.
“The Gamma4 Phase 2 launch of the intermediate nail, RC lag screw, and anti-rotation clip is sure to provide additional support to surgeons for treatment of challenging hip fractures,” said Dr. Sanjit Konda, Director of Trauma at NYU Langone Orthopedics and Jamaica Hospital Medical Center, and Chairman of Orthopedic Surgery at MediSys Health Network. “Intertrochanteric hip fractures with subtrochanteric extension can now be treated with a distally targeted intermediate nail. Hip fractures with involvement of the basicervical neck region can be provisionally stabilized with the anti-rotation clip and sleeve during lag screw insertion. The Phase 2 launch of these additional Gamma4 features will expand the surgeon's toolbox and add to an already best-of-class nailing system.”
About Stryker
Stryker is a global leader in medical technologies and, together with its customers, is driven to make healthcare better. The company offers innovative products and services in MedSurg, Neurotechnology, Orthopaedics and Spine that help improve patient and healthcare outcomes. Alongside its customers around the world, Stryker impacts more than 130 million patients annually. More information is available at www.stryker.com.
Dr. Sanjit Konda is a paid consultant of Stryker Trauma and Extremities. The opinions expressed by Dr. Sanjit Konda are those of Dr. Sanjit Konda and not necessarily those of Stryker. Individual experiences may vary.
A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery.
The information presented is intended to demonstrate the breadth of Stryker’s product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any of Stryker’s products. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your sales representative if you have questions about the availability of products in your area.
G4-FL-2, 11-2023
References
- Internal Report № D0000093463, Rev AC, Schönkirchen, Germany
- Internal Report № D0000245312, Rev AE, Schönkirchen, Germany
- G4-ST-1, Rev-3, 07-2023
- Internal Report № D0000250608. Rev AA, Schönkirchen, Germany