LIÈGE, Belgium & GRENOBLE, France--(BUSINESS WIRE)--PDC*line Pharma, a clinical stage biotech company developing a new class of potent and scalable active immunotherapies for cancer, announces today the selection of the PDC*neo+ project for funding by the Walloon region and BioWin, the health cluster for Wallonia. This is the result of a call for proposals on R&D projects in the field of Advanced Therapy Medicinal Products (ATMP). Members of the project consortium will receive €8.1M ($8.9M) in funding, including €4.7M ($5.1M) for PDC*line Pharma. The total budget is €12.5M ($13.7M).
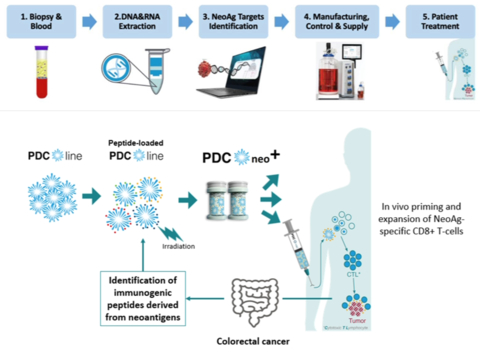
The project aims to develop PDC*neo+, a personalized therapeutic vaccine for colorectal cancer using PDC*line Pharma's innovative PDC*line technology. Targeting unique neoantigens in each colorectal cancer patient, PDC*neo+ represents a groundbreaking approach in cancer immunotherapy.
Globally, colorectal cancer (CRC) is among the most prevalent and deadly cancers, with a high recurrence rate post-surgery and chemotherapy. PDC*neo+ is designed as an adjuvant treatment to prevent relapses in high-risk patients with stages II, III and certain stage IV CRCs. Its action post-chemotherapy potentially makes it a pioneering treatment in CRC.
This project is a collaborative effort involving several key partners. OncoDNA contributes its expertise in personalized medicine and liquid biopsies, focusing on neoantigen identification and circulating tumor DNA analysis. salamanderU is developing a compact isolator for decentralized vaccine production to bring manufacturing closer to the clinical centers. Academic centers UCLouvain – IREC (Institut de Recherche Expérimentale et Clinique)/MIRO (Molecular Imaging, Radiotherapy and Oncology) and ULB-BCTL (Breast Cancer Translational Research Laboratory) offer essential support in translational research and clinical trial execution.
The main goal of the project is to confirm the clinical feasibility and safety of PDC*neo+ in a phase I trial. This initiative is expected to have a significant impact on CRC patient care, alongside broader economic and societal benefits. Over its three-year duration, across all partners, it is estimated to create or maintain around 34 full-time jobs.
“We are very happy that our project has been selected by the Walloon region. The subsidies and collaborative effort of the partners in the consortium will speed-up the launch of a first phase I clinical trial with PDC*neo+, the personalized vaccine based on PDC*line cells loaded with neoantigens to treat CRC patients, positioning our technology in the very promising field of neoantigen-based cancer vaccines,” said Eric Halioua, CEO of PDC*line Pharma.
“We are very thrilled to be part of this innovative project and clinical trial. It acknowledges our recent proficiency in the discovery of neoantigens and our robust experience in liquid biopsy monitoring by detection of circulating tumor DNA (ctDNA)," said Jean-Pol Detiffe, founder and chief strategy and innovation officer at OncoDNA.
“We are very enthusiastic about participating in this collaborative project and bringing the production of PDC*Neo+ closer to the patient. Through this project, salamanderU strengthens its position as a designer and manufacturer of innovative isolators for the manufacture of ATMP,” said Claude Dedry, CEO of salamanderU.
“We are very pleased and motivated to take part in this exciting and collaborative project. With our expertise, we will contribute to characterizing the immunogenicity of colorectal cancers and to the development and recruitment of patients for this clinical trial,” said Marc Van den Eynde and Jean-Pascal Machiels, oncologists at UCLouvain (Cliniques universitaires St-Luc).
“We are delighted to be involved in this interesting collaborative project. Leveraging our expertise in various ‘omics’ technologies, such as single-cell RNA sequencing and spatial transcriptomics, we will delve into the tumor microenvironment of colorectal cancers. Our objective is to develop predictive biomarkers for vaccine response and actively contribute to patient recruitment for the clinical trial," said Christos Sotiriou, head of the BCTL at ULB and Francesco Sclafani, gastrointestinal oncologist and clinical researcher at Institut Jules Bordet, Hopital Universitaire de Bruxelles (HUB) and Bordet Cancer Research Laboratory.
About PDC*line Pharma
Founded in 2014 as a spin-off of the French Blood Bank (EFS), PDC*line Pharma is a Belgian-French clinical-stage biotech company that develops an innovative class of active immunotherapies for cancers, based on a GMP-grade allogeneic therapeutic cell line of Plasmacytoid Dendritic Cells (PDC*line). PDC*line is much more potent than conventional dendritic cell-based vaccines in priming and boosting antitumor antigen-specific cytotoxic T-cells, including the T-cells specific for neoantigens, and is synergistic with checkpoint inhibitors. The technology can potentially be applied to any type of cancer. Following a first-in-human phase I feasibility study in melanoma, PDC*line Pharma is now focused on the development of PDC*lung01, its candidate for Non-Small-Cell Lung Cancer (NSCLC), currently in phase I/II trials, and PDC*neo with neoantigens, in preclinical development. The company has a staff of 42, with an experienced management team. It has raised more than €61M in equity and non-dilutive funding. In March 2019, PDC*line Pharma granted the LG Chem Life Sciences company an exclusive license in South Korea and an exclusive option in other Asian countries for the development and commercialization of the PDC*lung01 cancer vaccine for lung cancer. The total deal is worth €108M, plus tiered royalties on net sales in Asia.
www.pdc-line-pharma.com
About OncoDNA
OncoDNA is a leading genomic and theranostic company specializing in precision medicine for the treatment of cancer and genetic diseases. The company helps clinicians, academic researchers, and biopharma companies to outsmart molecular complexity with the mission of delivering the promise of precision medicine. The company not only provides clinical guidance for the treatment and real-time monitoring of late-stage cancer patients, but also supports research and drug development in cancer and genetic diseases. Since its early days in 2012, OncoDNA grew into a corporate group of companies with world-renowned expertise. The Group offers a unique portfolio that combines NGS services, biomarker testing, data analysis software and clinical decision support tools. OncoDNA is headquartered in Belgium, and its entities – BioSequence and IntegraGen – are based in Spain and France. OncoDNA employs over 115 employees across 9 countries, works with an international network of 35 distributors and collaborates with European-based accredited laboratories.
OncoDNA is keen to meet laboratory partners and oncologists who would like to benefit from our expertise and work in partnership with us for the benefit of oncologists and their patients.
Visit www.oncodna.com
About salamanderU
salamanderU is a technology company made up of a multidisciplinary team of business experts in the life sciences, pharmaceutical engineering and IT industries. We enhance our clients’ competitive advantage by increasing the robustness and the efficiency of their operational pharmaceutical processes. salamanderU develops and commercializes both technical and digital solutions: technical with acrylic resin isolators, and digital with voice-controlled electronic batch record and manufacturing execution system software.
www.salamanderu.com
About UCLouvain IREC/MIRO and Cliniques universitaires Saint-Luc
UCLouvain is one of the most important universities in Belgium. The IREC-MIRO-Oncology laboratory (UCLouvain) and the Cancer and Hematology Roi Albert II Institute at Cliniques universitaires Saint-Luc have expertise in clinical, translational and fundamental research in oncology. Through the conduct of several academic studies, we have characterized the tumor microenvironment of several cancers and investigated the effectiveness of different combinations of targeted and immune therapies. Our phase 1 research unit is internationally recognized in the investigation of innovative therapies such as novel immune and genetic treatments.
www.uclouvain.be
About IJB
The Institut Jules Bordet (IJB) is the only Comprehensive Cancer Centre in Belgium and is part of the Hopital Universitaire de Bruxelles (HUB) and of the Université Libre de Bruxelles (ULB). The strength of IJB is built upon its integration of three missions: excellence of care, innovative research and a high level of education. The Institute has participated in the creation of several international networks, including the European Organization for Research and Treatment of Cancer (EORTC), the Breast International Group (BIG), Accelerating Oncology Drug Development and Innovative Strategies in Clinical Trials (Oncodistinct) and the Organization of European Cancer Institutes (OECI). The Breast Cancer Translational Research Laboratory (BCTL), headed by Prof. Sotiriou, is an academic laboratory at the ULB faculty of medicine located within the Institut Jules Bordet. The main research focus of BCTL consists of improving the molecular understanding of breast cancer biology, disease dissemination and progression using state-of-the art ‘omics’ technologies as well as identifying novel prognostic and predictive biomarkers. The ultimate goal of this laboratory is to accelerate the translation into the clinic of basic science discoveries dedicated to breast cancer.
www.bordet.be