WOBURN, Mass.--(BUSINESS WIRE)--OSSIO, Inc., a fast-growing orthopedic fixation technology company, on Friday received “breaking” news from the U.S. Food and Drug Administration (FDA):
The FDA has cleared the company’s OSSIOfiber® bio-integrative fixation technology for use in orthopedic surgery for children and adolescents needing bone fractures fixed, osteotomies, or fusions. As a result, OSSIOfiber Compression Screws and Trimmable Fixation Nails may now be used in children from age two to 21 years in standard clinical practice.
‘Viable and desirable alternative’
“Using OSSIOfiber implants in children with bone fractures represents a viable and desirable alternative to implantation of traditional metal pins and screws, which in many situations need to be removed in a second surgical procedure,” explained David Lin, M.D., FAAOS, a leading pediatric orthopedic surgeon who practices at The Pediatric Orthopedic Center in northern New Jersey, near New York. “Another operation creates additional risk of complications while driving incremental healthcare costs, not to mention causing high levels of anxiety among most young patients and their parents.”
“When I believe a patient will benefit from an OSSIOfiber implant over a metal one, I’ll offer it,” he added.
“OSSIOfiber implants are strong like metal implants,” Dr. Lin continued, “with the added advantage of predictably becoming completely incorporated into the patient’s own bone within a reported two years, leaving no permanent foreign material behind. Practically speaking, their appeal is profound: During consultations with injured children and their concerned parents, the mere mention of avoiding a second surgery to repair the same fracture typically elicits an enthusiastically favorable response, as you would expect.”
“Looking ahead,” he concluded, “I predict bio-integrative implants will be the standard solution for orthopedic fixation among most children and adults in the coming years as OSSIO’s offering expands and more people, especially those in the medical community, learn more about it.”
‘Evolution of orthopedic surgery’
Mark E. Solomon, DPM, Director of the Pediatric Foot & Ankle Fellowship and a partner of Dr. Lin’s at The Pediatric Orthopedic Center, provided his perspective on OSSIO’s innovative fixation technology:
“The emergence of OSSIOfiber implants a few years ago marks an inflection point in the evolution of orthopedic surgery that my colleagues and I in the United States are keen to incorporate into our practice of what is already a highly evolved surgical subspecialty.
“On the heels of the technology’s FDA clearance for use in pediatric patients, I’m especially excited for OSSIO this week as the results of a clinical study we conducted of their bio-integrative implants in children treated at our center will be presented at IPOS 2023” –– this year’s annual meeting of the International Pediatric Orthopedic Society from Dec. 5–9 in Orlando, Fla.
The single-center study, conducted from June 2022 to June 2023 and titled “Orthopedic Fixation of Skeletally Immature Ankle Fractures in Pediatric Populations Using Bio-Integrative Implants,” was designed to “explore the outcomes of transitional ankle fractures after operative stabilization with bio-integrative versus metal alloy fixation devices.”
‘Comparable to metal-alloy devices, while offering advantages’
Shared in an e-poster today at IPOS 2023 and with hundreds more orthopedic surgeons from the United States and other countries who treat pediatric patients, typically defined as 2–21 years of age, the study’s results “suggest that bio-integrative fixation devices provide radiographic fracture healing rates comparable to metal-alloy devices in treating transitional ankle fractures, while offering advantages in terms of complication rates, re-operation rates, cost-efficiency for patients, and quality of life.”
They also “demonstrate bio-integrative fixation devices’ viability as an alternative to metal screws, most significantly, by saving a child from a second surgery,” the poster concludes.
Dr. Solomon is scheduled to present on his center’s OSSIOfiber study results at ACFAS 2024 –– next year’s annual meeting of the American College of Foot and Ankle Surgeons from Feb. 1–4 in Tampa, Fla. –– on Feb. 2. Meanwhile, he offered his view on their significance:
“My excitement about our contribution to the growing body of evidence on OSSIOfiber implants stems largely from the fact that this innovation in material science addresses the primary issue with fixing foot and ankle fractures in children –– namely, that their bones are still developing as they continue to grow into young adults. Because OSSIO’s medical technology naturally integrates with native bone, they don’t need to be removed, saving our pediatric patients from a second surgery.”
‘Expanding portfolio of OSSIOfiber implants’
“With this highly anticipated clearance, OSSIO enters an important segment of orthopedic surgery,” said OSSIO’s chief executive officer, Brian Verrier, “adding to our expanding portfolio of OSSIOfiber implants for the fixation of bone fractures and soft tissue injuries throughout the body in various clinical scenarios –– now including the treatment of fractures in children as young as two years old. Our innovation promises to be a gamechanger for clinicians, kids and parents dealing with the challenge of fixing fractured bones.”
The FDA’s approved instructions for the use of these bio-integrative implants in pediatric patients follows:
- The OSSIOfiber compression screw product family is now “indicated for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis, and bone grafts, of the upper extremity, fibula, knee, ankle and foot in the presence of appropriate brace and/or immobilization in adults and children (2–12 years) and adolescents (12–21 years) in which growth plates have fused or in which growth plates will not be crossed by fixation.”
- The OSSIOfiber fixation nail product family is now “indicated for maintenance of alignment and fixation of bone fractures, osteotomies, arthrodesis and bone grafts in the presence of appropriate additional immobilization (e.g. rigid fixation implants, cast, brace) in adults and children (2–12 years) and adolescents (12–21 years) in which growth plates have fused or in which growth plates will not be crossed by fixation.”
Intelligent Bone Regeneration Technology
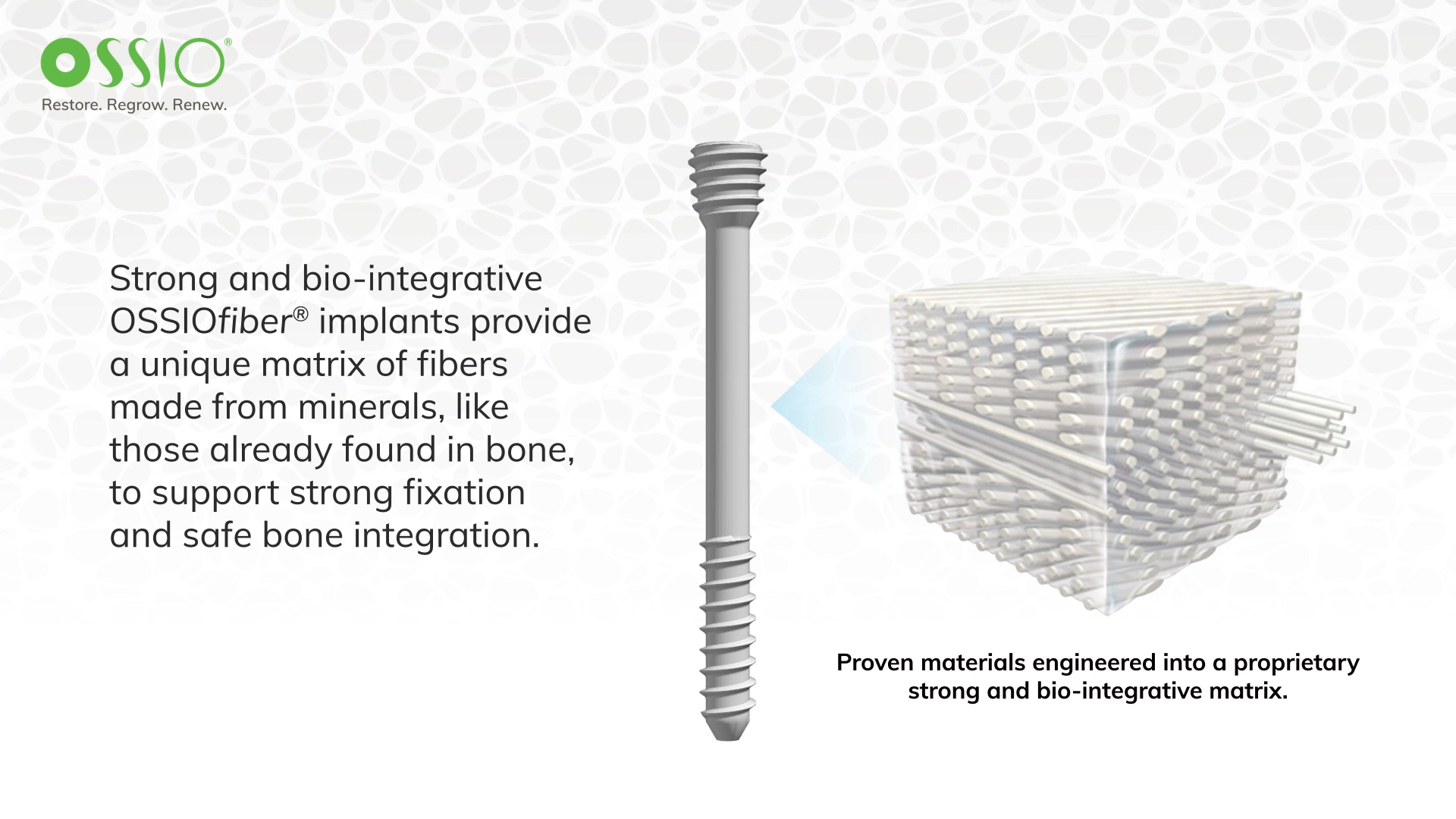
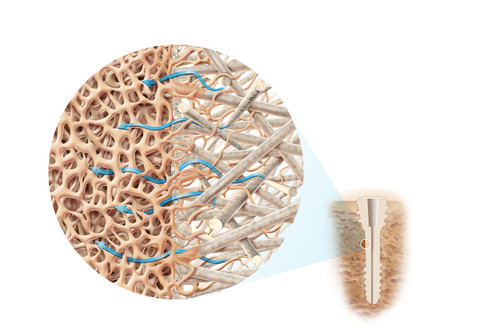
All OSSIO implants are made with OSSIOfiber Intelligent Bone Regeneration Technology, a breakthrough in fixation material that provides the first credible solution to the shortcomings of permanent metal hardware, conventional absorbable and allograft implants, combining unparalleled mechanical strength and natural bone healing in a non-permanent implant.
Made from a proprietary mineral fiber matrix, their bio-integrative material properties provide surgeons with a more biologically friendly way to restore patients’ stability and mobility while leaving nothing permanent behind.
Designed for rapid bone in-growth, regeneration and replacement, OSSIOfiber Intelligent Bone Regeneration Technology is a first-of-its-kind implant material stronger than cortical bone. It is engineered to provide the strength required for functional fixation while allowing for full integration into patients’ native anatomy without adverse biological response. It can address many surgical applications through the manufacturing of endless implant designs, including nails, screws, staples, anchors and plates.
Looking ahead, OSSIO intends to pursue multiple applications for OSSIOfiber implants in all major segments of orthopedics, such as foot and ankle, hand and wrist, upper extremity and trauma, sports medicine and reconstruction.
Notably for U.S. healthcare providers and payors, OSSIOfiber implants utilize existing reimbursement and surgical techniques.
About OSSIO
OSSIO is an orthopedic fixation company committed to transforming the orthopedic experience for patients, physicians, providers and payors. Founded in 2014, the company’s vision is to provide the first credible replacement to metal implants in the multibillion-dollar global orthopedic fixation market with OSSIOfiber Intelligent Bone Regeneration Technology.
For more information on OSSIOfiber implants and OSSIO, which has development headquarters in Caesarea, Israel, and commercial headquarters in Woburn, Mass.: www.ossio.io.
__________________________
NOTE: Interviews with clinicians, children and caregivers who have experience with the product can be arranged for members of the news media. Additionally, animation and other graphic assets showing how the product works are included with this multimedia news release and may be included in news coverage and social posts with appropriate credit to OSSIO as their source.
DISCLAIMER: Forward-looking statements contained herein are based on estimates and assumptions of OSSIO management and are believed to be reasonable, though they are inherently uncertain and difficult to predict.