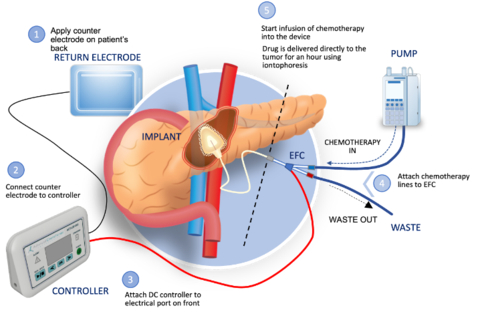
CARY, N.C.--(BUSINESS WIRE)--Focal Medical, Inc., a privately held, biopharmaceutical company developing novel precision therapeutic products today announced successful completion of the nonclinical development phase of Focal Medical’s Implantable Iontophoresis Chemotherapy Delivery Device with Gemcitabine (ACT-IOP-003).
ACT-IOP-003 is a novel implanted precision therapeutic system intended to safely deliver multiple doses of gemcitabine (an FDA approved therapeutic agent) locally and actively into the pancreas using non-circulatory pathways. The completion of animal studies is a significant milestone in the development program in support of the efficacy, safety, and tolerability of ACT-IOP-003 for the treatment of pancreatic cancer. Focal Medical expects to initiate a pancreatic cancer clinical development program in 2024.
The pivotal GLP study was informed by unprecedented efficacy results in an orthotopic patient derived xenograft murine model of human pancreatic cancer1, which was followed by a battery of pilot studies exploring safety and tolerability ranges in two separate healthy pancreas large animal models (canine and porcine). The final GLP study successfully demonstrated the safety and physiologic response to treatment of the final and clinical version of ACT-IOP-003 when employed under clinically relevant implant methods and treatment conditions in the pig.
“The completion of the development of our precision therapeutic system to deliver gemcitabine directly to the pancreas is an extraordinarily important milestone for Focal Medical,” commented Michael Aldridge, Focal Medical Chief Executive Officer.
“The system’s ability to precisely target the pancreas and actively deliver gemcitabine via non-circulatory pathways, with little systemic exposure is potentially enormously promising for patients diagnosed with locally advanced non-resectable pancreatic cancer. We look forward to initiating our clinical program in 2024.”
Pivotal Study Design and Outcomes
Data from Focal Medical’s prior nonclinical studies in a well characterized healthy pancreas porcine model provided the basis for the design of the pivotal GLP study, the goals of which were developed in consultation with FDA, to support the safety and tolerability of ACT-IOP-003 for the treatment of locally advanced, non-resectable pancreatic cancer in humans.
Animals were laparoscopically implanted with the ACT-IOP-003 system and subjected to twice weekly transpancreatic iontophoresis treatment with gemcitabine over the course of eight (8) weeks, beginning post-operative Week 1. Five (5) animals successfully completed the sixteen (16) prescribed treatments and survived to scheduled termination.
Qualified study animals remained in good clinical health, and no adverse events were encountered in these animals throughout the in-life duration. Assessments of overall animal health inclusive of daily clinical observations, weekly veterinary physical examinations, weekly body weights, and weekly body condition scores suggested that all full-term animals remained in excellent clinical health and ideal body condition throughout the study. No implant- or treatment-mediated trends in abnormal clinical pathology parameters were identified, and no changes in amylase or lipase levels were observed. Together, these results indicated no pancreatic injury or impairment of pancreatic function.
The gross findings at necropsy were representative of typical and expected changes for the device type, implantation procedure, implant location, and survival time point; there were no gross findings suggestive of a safety concern for the chronic implantation and use of the ACT-IOP-003 System. The implantable drug reservoir of the ACT-IOP-003 System was considered to have minimal to no histological reaction. There were no histological indicators of systemic toxicity in all non-target and downstream organ systems and tissues.
Based on the data obtained in this study, the physiological response to twice weekly transpancreatic iontophoresis treatment with gemcitabine utilizing the system demonstrated a safe method for chemotherapy drug delivery to the pancreas under clinically relevant conditions.
These results are consistent with additional biocompatibility studies that were conducted in accordance with ISO 10993-1 and FDA Guidance. The results of these studies indicated that the ACT-IOP-003 device is not cytotoxic, not a sensitizer, not irritating, and not acutely toxic.
Given the nature and history of the component materials and the results of a program of biocompatibility studies, the ACT-IOP-003 device is considered to be biocompatible for the planned clinical studies and no adverse effects are anticipated for patients.
About Pancreatic Cancer
The incidence of pancreatic cancer in the US is 62,000 cases per year. It is a devastating disease and the second leading cause of cancer death behind lung cancer. Pancreatic cancer is a difficult cancer to detect and treat. It often does not cause symptoms until it is in its later stages, when it is more difficult to cure. Following diagnosis, pancreatic cancer has a 5-year survival rate of only 12%.2 Treatment primarily consists of intravenous chemotherapy. On diagnosis, only 15% of patients are eligible for surgical removal of the tumor. The average per patient cost of care is $280,433 in the first year of treatment and $312,077 in the second year.3
About Focal Medical
Focal Medical, Inc. is a privately held, biopharmaceutical company developing novel therapeutic products based on its innovative and patent protected precision medicine platform. Focal Medical’s lead product is a precision therapeutic delivering gemcitabine (an FDA approved chemotherapeutic) actively and directly to the pancreas by non-circulatory pathways to treat pancreatic cancer.
Focal Medical is expanding its product focus into therapies for other solid tumors and genomic medicine products. Its products utilize its innovative energy-based precision medicine system.
Focal Medical’s patented iontophoresis delivery system enables the internal, site-specific delivery of therapeutics actively, directly, and selectively to the target organ using non-circulatory pathways. The technology thus addresses certain significant challenges and limitations of traditional systemic drug delivery including systemic toxicity and barriers to therapeutic effect.
1 “Local iontophoretic administration of cytotoxic therapies to solid tumors”, Byrne JD et al. Sci Transl Med. 2015 Feb 4;7(273).
2 https://seer.cancer.gov/statfacts/html/pancreas.html
3 2020 GI Cancers Symposium: Cost of Pancreatic Cancer Care Over Time – The ASCO Post