ZUG, Switzerland--(BUSINESS WIRE)--Galderma announced positive data from three pivotal phase III trials in atopic dermatitis and prurigo nodularis – ARCADIA 1 and 2 and OLYMPIA 1, respectively. The late-breaking data were presented today at the 2023 European Academy of Dermatology and Venereology (EADV) congress in Berlin. The ARCADIA 1 and 2 trials met their co-primary and all key secondary endpoints, demonstrating that nemolizumab significantly reduced skin lesions and itch in patients with atopic dermatitis, with clinically meaningful results for itch improvement as early as week 1.1
“While atopic dermatitis and prurigo nodularis are distinct diseases, their commonality lies in the severe, persistent itch, which leads to poor quality sleep and negatively impacts mental health. These important results highlight nemolizumab’s potential to be an effective and convenient treatment for the millions of people globally who live with these conditions.”
FLEMMING ØRNSKOV, M.D., MPH
CHIEF EXECUTIVE OFFICER
GALDERMA
Results from the ARCADIA 1 and 2 trials showed that nemolizumab significantly improved skin lesions and itch in adolescent and adult patients with moderate to severe atopic dermatitis, compared to placebo (both administered with background topical corticosteroid therapy or topical calcineurin inhibitors). In both trials, adolescent and adult patients treated with nemolizumab showed clinically and statistically significant improvements in co-primary endpoints, compared to placebo after 16 weeks of treatment. Results across both trials showed:1
- 35.6% and 37.7% of nemolizumab-treated patients in ARCADIA 1 and 2, respectively, reached clearance or almost-clearance of skin lesions when assessed using the investigator’s global assessment (IGA) score, compared to 24.6% and 26.0% in the placebo group (p<0.0006, p=0.001).
- 43.5 percent and 42.1% of nemolizumab-treated patients in ARCADIA 1 and 2, respectively, achieved a 75% reduction in the Eczema Area and Severity Index (EASI), compared to 29.0% and 30.2% in the placebo group (p<0.0001, p=0.0011).
The trials also met all key secondary endpoints, with 48.6% and 48.1% of nemolizumab-treated patients achieving an at least four-point reduction in itch in ARCADIA 1 and 2, respectively, as measured by the peak-pruritus numerical rating scale (PP-NRS) score, compared to 20.5% and 20.6% in the placebo group (p<0.0001), within 16 weeks of treatment. Statistically significant results at week 16 and earlier time points also show nemolizumab’s rapid onset of action on itch and sleep disturbance. Nemolizumab was well tolerated, and its safety profile was consistent between the ARCADIA 1 and 2 studies.1
Data from the phase III OLYMPIA 1 trial bolster evidence for nemolizumab’s rapid onset of action on itch and reduction of skin lesions in patients with prurigo nodularis
In the phase III OLYMPIA 1 trial, nemolizumab as a monotherapy significantly improved itch and skin lesions in adult patients with moderate to severe prurigo nodularis, compared to placebo. Patients treated with nemolizumab monotherapy (without background topical corticosteroids or topical calcineurin inhibitors) showed clinically and statistically significant improvements in both primary endpoints, compared to placebo after 16 weeks of treatment, providing independent confirmation of results from the phase III OLYMPIA 2 trial:2
- 58.4% of nemolizumab-treated patients achieved an at least four-point reduction in itch, as measured by the PP-NRS score, compared to 16.7% in the placebo group (p<0.0001).
- 26.3% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions, when assessed using the IGA score, compared to 7.3% in the placebo group (p<0.0001).
The trial also met all key secondary endpoints confirming rapid onset of action on itch as early as week 4 (41.1% compared to 6.3% in the placebo group; p<0.0001), and improvements in itch and skin lesions were observed up to week 24. Nemolizumab was well tolerated, and its safety profile was consistent with OLYMPIA 2 trial results.2
“Through targeting the neuroimmune cytokine IL-31, these phase III data demonstrate that nemolizumab significantly and quickly improves three of the most burdensome symptoms of these conditions: itch, skin lesions, and sleep disturbance.”
PROFESSOR JONATHAN SILVERBERG, MD, PHD, MPH
LEAD ADVISOR AND INVESTIGATOR OF ARCADIA CLINICAL TRIALS
DIRECTOR OF CLINICAL RESEARCH
THE GEORGE WASHINGTON UNIVERSITY SCHOOL OF MEDICINE AND HEALTH SCIENCES, WASHINGTON, DC
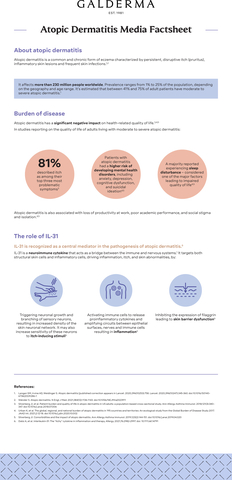
Nemolizumab is an investigational monoclonal antibody specifically designed to target the IL-31 receptor and inhibit IL-31 signaling. IL-31 plays a key role in multiple disease mechanisms in both atopic dermatitis and prurigo nodularis.5-10 This includes directly addressing the source of itch, which causes sleep disturbance and negatively impacts quality of life outcomes.3,4,7-13 Results from the ARCADIA and OLYMPIA phase III trials will be submitted to selected health authorities around the world. Nemolizumab was granted Breakthrough Therapy designation by the U.S. Food and Drug Administration (FDA) in December 2019 for the treatment of itch associated with prurigo nodularis.
Media can find more information about atopic dermatitis and prurigo nodularis in this media toolkit.
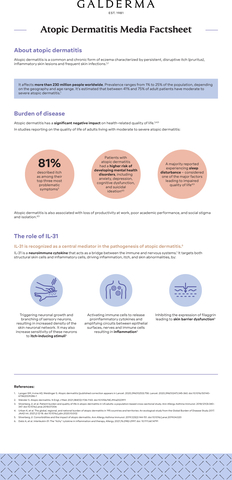
About atopic dermatitis
Atopic dermatitis is a common and chronic form of eczema characterized by persistent, disruptive itch (pruritus), inflammatory skin lesions, and frequent skin infections, affecting 230 million people worldwide.5,14 Reported prevalence of atopic dermatitis varies greatly, ranging from 1% to 25% of the population depending on the geography and age range.4,12,13
About prurigo nodularis
Prurigo nodularis is a debilitating chronic skin condition characterized by thick skin nodules covering large body areas and associated with intense itch (pruritus).6,15,16 Prurigo nodularis affects an estimated 72 out of every 100,000 adults aged 18 to 64 in the United States. It is more common in middle-aged women and, disproportionately, people of African descent.6,17
About nemolizumab
Nemolizumab is in clinical development for the treatment of atopic dermatitis and prurigo nodularis in many countries around the world. It was initially developed by Chugai Pharmaceutical Co., Ltd., and subsequently licensed to Galderma in 2016 – worldwide except Japan and Taiwan. In Japan, nemolizumab is approved for the treatment of pruritus associated with atopic dermatitis and is in development for prurigo nodularis.
About the ARCADIA clinical trial program
The ARCADIA program included two identical, pivotal phase III clinical trials which enrolled more than 1,500 patients – ARCADIA 1 and ARCADIA 2. The trials evaluated the efficacy and safety of nemolizumab administered subcutaneously every four weeks, compared to placebo, in adolescent and adult patients with moderate to severe atopic dermatitis. Patients were evaluated after a 16-week initial treatment period. Clinical responders continued to a maintenance treatment period for up to an additional 32 weeks thereafter. Clinical non-responder patients at week 16 could enroll into a long-term extension study.
About the OLYMPIA 1 trial
OLYMPIA 1 was a randomized, double-blind, placebo-controlled phase III clinical trial designed to assess the efficacy and safety of nemolizumab monotherapy compared with placebo in patients aged at least 18 with prurigo nodularis after a 16-week treatment period. The trial also assessed the pharmacokinetics and immunogenicity of nemolizumab compared to placebo. OLYMPIA 1 included 286 patients with moderate to severe prurigo nodularis.
About Galderma
Galderma is the emerging pure-play dermatology category leader, present in approximately 90 countries. We deliver an innovative, science-based portfolio of premium flagship brands and services that span the full spectrum of the fast-growing dermatology market through Injectable Aesthetics, Dermatological Skincare and Therapeutic Dermatology. Since our foundation in 1981, we have dedicated our focus and passion to the human body’s largest organ – the skin – meeting individual consumer and patient needs with superior outcomes in partnership with healthcare professionals. Because we understand that the skin we’re in shapes our lives, we are advancing dermatology for every skin story. For more information, visit www.galderma.com.
References:
- Silverberg J, et al. Nemolizumab improves skin lesions, itch and sleep disturbance in patients with moderate-to-severe atopic dermatitis: Results from two identical phase 3 multinational studies (ARCADIA 1 and ARCADIA 2). Late-breaking abstract presented at EADV 2023.
- Stander S, et al. Nemolizumab monotherapy improves itch and skin lesions in patients with moderate-to-severe prurigo nodularis: Results from a global phase 3 trial (OLYMPIA 1). Late-breaking abstract presented at EADV 2023.
- Pereira MP, et al. Chronic nodular prurigo: clinical profile and burden. A European cross-sectional study. J Eur Acad Dermatol Venereol. 2020;34(10):2373-2383. doi:10.1111/jdv.16309.
- Silverberg JI, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340-347. doi:10.1016/j.anai.2018.07.006.
- Langan SM, et al. Atopic dermatitis [published correction appears in Lancet. 2020;396(10253):758] Lancet. 2020;396(10247):345-360. doi:10.1016/S0140-6736(20)31286-1.
- Williams KA, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14(1):67-77. doi:10.1080/17512433.2021.1852080.
- Nemmer JM, et al. Interleukin-31 signaling bridges the gap between immune cells, the nervous system and epithelial tissues. Front Med (Lausanne). 2021;8:639097. doi:10.3389/fmed.2021.639097.
- Wang F, Kim BS. Itch: a paradigm of neuroimmune crosstalk. Immunity. 2020;52(5):753-766. doi:10.1016/j.immuni.2020.04.008.
- Zhang Q, et al. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008;19(5-6):347-356. doi:10.1016/j.cytogfr.2008.08.003.
- Tsoi LC, et al. Transcriptomic characterization of prurigo nodularis and the therapeutic response to nemolizumab. J Allergy Clin Immunol. 2021;S0091-6749(21)01557-8. doi:10.1016/j.jaci.2021.10.004.
- Gwillim EC, Nattkemper L, Yosipovitch G. Impact of Itch on Sleep Disturbance in Patients with Prurigo Nodularis. Acta Derm Venereol. 2021;101(3):adv00424. doi:10.2340/00015555-3778. PMID: 33704503.
- Urban K, et al. The global, regional, and national burden of atopic dermatitis in 195 countries and territories: An ecological study from the Global Burden of Disease Study 2017. JAAD Int. 2021;2:12-18. doi:10.1016/j.jdin.2020.10.002.
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123(2):144-151. doi:10.1016/j.anai.2019.04.020.
- Ständer S. Atopic dermatitis. N Engl J Med. 2021;384(12):1136-1143. doi:10.1056/NEJMra2023911.
- Elmariah S, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84(3):747-760. doi:10.1016/j.jaad.2020.07.025.
- Whang KA, et al. Prevalence of prurigo nodularis in the United States. J Allergy Clin Immunol Pract. 2020;8(9):3240-3241. doi:10.1016/j.jaip.2020.05.051.
- Huang AH, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140(2):480-483.e4. doi:10.1016/j.jid.2019.07.697.