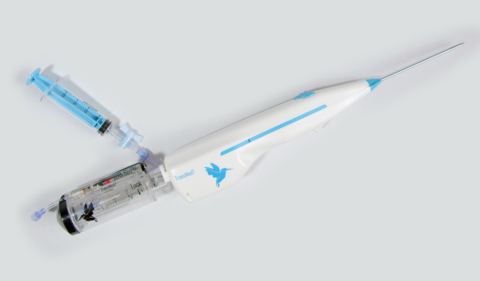
PORTOLA VALLEY, Calif.--(BUSINESS WIRE)--TransMed7, LLC announced today the first clinical use of VacuPac®, a new self-contained external vacuum-assist attachment with a SpeedBird Universal and a Concorde US from the SpeedBird and Concorde families of vacuum-assisted, Single Insertion – Multiple Collection (SIMC® ) breast biopsy devices. This follows the announcements in August 2022 and February 2023 of the first clinical uses of SpeedBird and Concorde biopsy devices, as part of the commercial launch of TransMed7’s vacuum-assisted, SIMC® breast biopsy devices. Procedures using VacuPac® devices on the biopsy instruments were performed by Dr. Michael Berry, distinguished surgeon and leader in the American Society of Breast Surgeons (ASBrS) at the Margaret West Comprehensive Breast Center in Germantown, TN.
Dr. Berry commented on his results: “As I widen my adoption of TransMed7’s breast biopsy devices within the SpeedBird and Concorde families, I foresee clinical and lesion factors where I may favor one of the devices over another. The novel scoopula feature of the Concorde devices for example, can be very helpful for small lesions. For routine biopsies I have found the Sparrow to be my go-to choice and the SpeedBird Universal will also fill that role in a comfortable form factor. I was especially excited to perform the first clinical cases using TransMed7’s VacuPac®, a new self-contained, tetherless external vacuum source attachment, coupled with a SpeedBird Universal and a Concorde Ultrasound for two successful cases.”
He added, “We previously reported that image-guided breast biopsies using TransMed7’s vacuum-assisted, SIMC® devices involve minimal set-up, are very easy to perform with their extreme ultrasound visibility and take only a few minutes to complete, start to finish. VacuPac® simplifies these procedures even further by completely eliminating suction tubing connected to separate canisters and vacuum pumps. Its built-in vacuum delivers whole solid cores into a removable container, separate from aspirated fluid, which is saved in its own integrated canister for cytologic analysis. The VacuPac® adds minimal weight to the biopsy device, is self-powered, quiet and simple to operate and enhances freedom of movement by eliminating all constraining tethers of suction tubing.”
Dr. Berry summarized, “I was pleased to confirm that, just as we reported with TransMed7 devices using standard wall suction or TransMed7’s VacuTower™, the ‘VacuPac®’ likewise enabled us with just a few passes to obtain large, full diameter specimens of high surgical quality, which again provided all of the lesion-specific information enabling our team to make a definitive diagnosis and patient-tailored plan.”
Dr. Ed Staren, President of TransMed7, stated, “It has been my wish to add a portable, self-contained vacuum source to the already very handy SpeedBird and Concorde devices as an option for a completely tetherless procedure, while preserving the ability to use external vacuum sources when desired. I give credit to the technical team for enabling this option by developing the VacuPac® concept into a successful reality. Therefore it gives me great satisfaction to announce the successful clinical utilization of our VacuPac® attachment for our minimally invasive breast biopsy devices. This milestone demonstrates that an attachable, disposable, vacuum-assist device may be utilized to support any of our Zero5® technology-based biopsy instruments in a convenient, tetherless form for the care and benefit of patients. We anticipate that VacuPac® will provide a useful option to support Zero5® technology in all of our next generation device platforms in their various applications all over the body. These include: Heron, Cardinal, Thunderbird and Phoenix in addition to its demonstrated use in SpeedBird and Concorde.”
Dr. James W. Vetter, TransMed7 Co-Founder and Chairman stated, “We specifically developed the single use, disposable VacuPac® as a separate device to keep the biopsy devices themselves simple and cost-effective, while giving specialists freedom to choose suction sources for any given case. Leveraging VacuPac®’s clinical successes and enthusiastic reception, we are expanding the lineup to include an even smaller version to match our Cardinal Fine Needle Core Biopsy Platform. VacuPac® and its variants in the pipeline demonstrate our commitment to provide full fluid management in all current and future Zero5®-based SIMC® devices whether totally untethered with VacuPac® or connected by tubing to wall suction or other sources including our own rechargeable battery-powered VacuTower™.”
The SpeedBird and Concorde device platforms are based on TransMed7’s patented Zero5® work element composed of a fused, single element constructed from 3 hypotubes and forming rotating twin cutter blades. This element gently penetrates, cores, severs, and provides a pathway to transport multiple tissue samples via a closed-circuit fluid management and vacuum system into a detachable chamber. Zero5® technology enables these devices to reliably and consistently obtain full-core, uniform-diameter samples with intact architecture from all of the various soft tissues. It was developed as an entirely new mechanism intended to deliver superior results while also lowering costs, and to facilitate ease of use for rapid learning curve and adoption.
Eugene H. Vetter, TransMed7’s Co-Founder and CEO, stated, “The successful use of these devices in their different forms and combinations now proves that our goal of exploiting our patented Zero5® technology to provide the world’s most extensive range of transformational, next generation, minimally invasive device platforms for biopsy procedures and interventional solutions is now reality. We will soon be providing these transformational devices to physicians so that their patients can finally look forward to this next generation of devices replacing the decades-old technologies currently being used on them, so stay tuned for more information from us shortly.”
About TransMed7, LLC -
TransMed7, LLC is a medical and technology based organization focused on the highly efficient development of innovative, minimally invasive medical devices aimed at providing new solutions for doctors and their patients. With particular expertise in oncologic, regenerative, and cardiovascular disease, TransMed7 and its team of clinicians, scientists, and engineers have developed a portfolio of next-generation platform devices that are expected to be market leaders in their targeted fields of medicine. TransMed7 accomplishes this through application of a wholly new approach in its business plan and structure, enabling these new transformational technologies rapid development through commercial manufacturing or where appropriate, handoff through acquisition to our strategic partners.
For more information about TransMed7 please visit our website at www.transmed7.com.