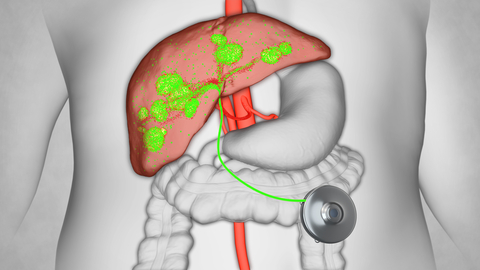
BOSTON--(BUSINESS WIRE)--Intera Oncology® Inc. has reintroduced Hepatic Artery Infusion (HAI) therapy to the market after its discontinuation in 2018 by the prior manufacturer dismayed patients and physicians. Hepatic Artery Infusion therapy is giving people with colorectal cancer that has spread to the liver and those with bile duct cancer confined to the liver new hope by delivering local chemotherapy directly to tumors while minimizing side effects elsewhere in the body.
Intera Oncology was founded by Jonathan Reis, M.D. and David Dove, M.D. who learned of the pump’s discontinuation from a New York Times article published five years ago. Drs. Reis and Dove had both been impressed with the clinical research supporting HAI therapy and had personally seen the benefits of the treatment on their friends and loved ones. Together, they made a commitment to building an entire company with an exclusive focus on bringing the pump back to market to ensure HAI therapy’s availability for all patients who need it.
For patients with colorectal cancer that has spread to the liver and those with bile duct cancer confined to the liver, HAI therapy has been shown in multiple independent clinical studies to improve survival and help prevent the recurrence of tumors. After Intera Oncology received FDA approval to manufacture the pump in 2021, the medical community quickly rallied in support of the pump’s return with nearly 50 cancer centers across the U.S.— including nine of the U.S. News & World Report’s top 10 in the nation—now offering HAI therapy to a growing number of patients.
“We regularly speak to survivors who attribute their improved quality of life and treatment success to HAI therapy, and it’s our mission to keep this treatment option available to potentially improve outcomes for countless more,” said Intera Oncology President and CEO Michael Gaisford.
Patient demand and self-referrals have been a key driver of adoption for the Intera 3000 HAI pump which fits in the palm of a hand and is surgically implanted into the abdomen wall. The pump is designed to deliver a continuous flow of chemotherapy directly to the hepatic artery that feeds metastatic tumors in the liver. This direct delivery mechanism allows for delivering 400 times the concentration of the drug into the tumors in the liver compared to standard IV administration. Patients who receive HAI therapy continue their normal daily activities outside of a hospital with minimal disruption to their lives.
One such patient, Marsha Semon, today a 48-year-old mother of two, received HAI therapy in 2017 and was able to keep up with her third-grader and kindergartener while getting treatment. Six years later, she is an advocate that encourages other colorectal cancer patients to consider the HAI pump to treat liver metastases. She says, “Thanks to Hepatic Artery Infusion therapy, patients like me are given an opportunity to continue living our lives for our children and families. It’s truly amazing that Intera Oncology as a company was created for the sole purpose of bringing back a treatment that is so desperately needed. There are so many patients who need to know about this.”
To learn more about HAI therapy, visit www.InteraOncology.com.
ABOUT INTERA ONCOLOGY: Intera Oncology® Inc. is a Boston-based medical device company founded in 2019 by two doctors with a singular vision for improving the survival of colorectal cancer and cholangiocarcinoma patients by ensuring access to Hepatic Artery Infusion (HAI) therapy. Dedicated to helping patients live longer and better lives by changing the course of cancer with HAI therapy, Intera Oncology manufactures the only FDA-approved pump for HAI therapy – the Intera 3000 Hepatic Artery Infusion Pump. Today, the treatment is used in nearly 50 cancer centers across the U.S., including nine of the U.S. News & World Report’s top 10 in the nation. Learn more at interaoncology.com and follow Intera on Twitter, LinkedIn and Facebook.
Editor’s Note: Patients and experts are available to share their HAI treatment stories. Contact intera@beyondfifteen.com or 949.733.8679.