MOUNTAIN VIEW, Calif.--(BUSINESS WIRE)--Aerin Medical Inc., a company dedicated to providing Ear, Nose and Throat (ENT) physicians with non-invasive solutions for the treatment of chronic nasal conditions, announced today that the Centers for Medicare and Medicaid Services (CMS) assigned physician and facility payment for a new Category I Current Procedural Terminology (CPT®) code that includes the use of VivAer® to repair nasal valve collapse (NVC) when performed in the office and outpatient surgical settings. The new code goes into effect on January 1, 2023.
“Actions by the AMA and CMS to establish and implement this new Category I code are an important validation of treatment with VivAer as a unique and distinct therapy for the repair of NVC,” said Matt Brokaw, CEO of Aerin Medical. “We are excited about the potential for improved access for the ENT physician community and millions of nasal airway obstruction sufferers in the U.S.”
The new code was implemented and assigned a national payment rate as part of CMS’ Calendar Year (CY) 2023 Medicare Physician Fee Schedule Final Rule and its CY 2023 Hospital Outpatient Prospective Payment System and Ambulatory Surgical Center Payment System Final Rule. CPT code 30469, repair of NVC with low energy, temperature-controlled (i.e., radiofrequency) subcutaneous/submucosal remodeling, was established by the American Medical Association (AMA) describing use of VivAer for this procedure. Because of the non-invasive nature of the treatment, the new code has been assigned a zero-day global period.
About VivAer
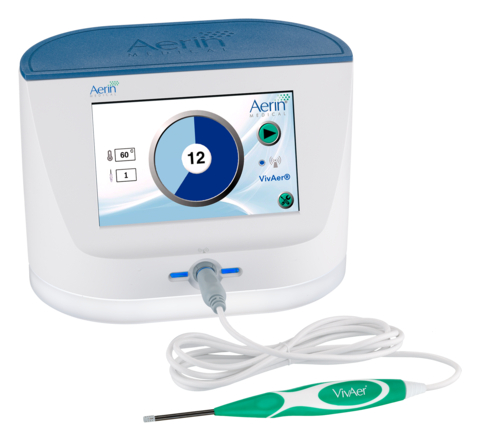
VivAer is a non-invasive technology that uses patented, temperature-controlled radiofrequency energy and is clinically demonstrated to provide long-term relief from nasal obstruction. VivAer features a thin, wand-like stylus that attaches to a console. The stylus is inserted via the nostril to gently remodel the nasal tissue and improve airflow. VivAer does not involve any cutting, freezing, or removal of nasal tissue or bone. Treatment with VivAer may be performed during an office visit under local anesthesia. Patients typically experience little discomfort with minimal to no downtime and are often able to return to normal activities immediately following treatment.* The VivAer Stylus received CE Mark in 2016 and FDA 510(k) clearance in December 2017. For more information, visit www.VivAer.com.
About Aerin Medical
Aerin Medical is a privately held, venture-backed company, with U.S. offices in California and Texas. Aerin’s mission is to provide ENT physicians with non-invasive solutions for the treatment of chronic nasal conditions. The company’s products, VivAer® for nasal airway obstruction and RhinAer® for chronic rhinitis, leverage Aerin’s proprietary temperature-controlled technology, which allows ENT physicians to reliably improve patients’ symptoms with in-office procedures performed with local anesthetic. More than 75,000 patients have been treated with Aerin Medical products to date. For more information, please visit www.aerinmedical.com and follow Aerin Medical on Facebook, Twitter, Instagram, LinkedIn and YouTube.
*Individual results may vary.