PETACH TIKVA, Israel--(BUSINESS WIRE)--Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CFBI), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address inflammatory, cancer and liver diseases, announced today that a poster entitled “Complete Response Induced by Namodenoson, an A3 Adenosine Receptor Agonist, in a Patient with Advanced Hepatocellular Carcinoma” will be presented at the American Association for the Study of Liver Diseases’ (AASLD) The Liver Meeting® at 1:00 pm on Monday, November 7, 2022 in Washington, D.C. The findings are published, Abstract 4413, in the October 2022 supplement of HEPATOLOGY, a premier peer-reviewed journal in the field of liver disease published on behalf of the AASLD.
Summary of Abstract:
The patient, a 61-year-old woman with hepatocellular carcinoma (HCC), the most common form of liver cancer, and moderate hepatic dysfunction Child-Pugh B (CPB7), participated in Can-Fite’s prior Phase II study. The patient was in the Namodenoson arm of the Phase II study and continued treatment with Namodenoson for 5 years under an Open Label Extension Program. Treatment is ongoing under a Compassionate Use Program established in Romania in August of 2022.
Highlights:
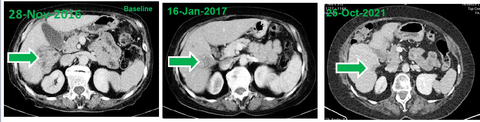
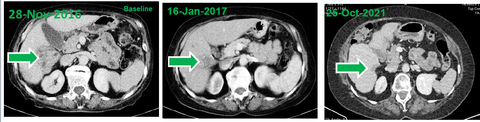
- At baseline, computed tomography (CT) scans demonstrated a large HCC tumor in the context of multifocal disease at baseline. Two treatment cycles later (e.g., after approximately 7 weeks) CT demonstrated shrinkage of the tumor mass that was consistent with a partial response.
- Within 4 years of treatment, disappearance of the tumor mass, ascites and peritoneal carcinomatosis was observed consistent with a complete response by RECIST 1.1 and mRECIST.
- The patient’s alanine transaminase (ALT) and aspartate aminotransferase (AST) levels were elevated at baseline (68 U/L and 44 U/L, respectively), and normalized after 1 treatment cycle. Normal ALT and AST levels were maintained for 5 years.
- Serum α-fetoprotein level was 47 ng/ml at baseline, declined to normal levels after 5 cycles of treatment, and reached 1.3 ng/mL at the time of complete response.
- No treatment-emergent adverse events were reported.
- At the time of reporting this case (5 years from treatment initiation), the response is ongoing as indicated by evaluation of liver functions and imaging studies.
Conclusion:
This case report demonstrates that treatment with Namodenoson can lead to a complete and durable response in patients with HCC and CPB7.
“A Complete Response of HCC in an advanced stage is rare, and we are pleased to report that under treatment with Namodenoson, this patient has now survived more the five years, returning to normal liver function with the disappearance of ascites and peritoneal carcinomatosis,” stated Can-Fite CEO Dr. Fishman. “We look forward to sharing our findings on this case with the community of hepatologists at The Liver Meeting in order to advance scientific knowledge and bring to market safe and effective treatments for liver cancer.”
Can-Fite’s pivotal Phase III study in patients with advanced liver cancer is open for patient enrolment and will recruit patients in Israel, the U.S., and five countries in Europe. If the study achieves its endpoint, the Company will be in a position to submit Namodenoson for approval with the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Namodenoson has Orphan Drug Status with both the FDA and EMA and Fast Track Status with the FDA. A registration plan has been submitted to and accepted by the FDA.
The Liver Meeting, which takes place in Washington, D.C. from November 4 – 8, 2022, brings together clinicians, associates, and scientists from around the world to exchange information on the latest research, discuss new developments in liver treatment and transplantation, and network with leading experts in the field of hepatology.
The HCC drug market is expected to reach $3.8 billion in 2027 in the G8 countries according to DelveInsight.
About Namodenoson
Namodenoson is a small orally bioavailable drug that binds with high affinity and selectivity to the A3 adenosine receptor (A3AR). Namodenoson is being evaluated for two indications, as a second line treatment for hepatocellular carcinoma in a pivotal Phase III study, and as a treatment for non-alcoholic steatohepatitis (NASH) in a Phase IIb study. A3AR is highly expressed in diseased cells whereas low expression is found in normal cells. This differential effect accounts for the excellent safety profile of the drug.
About Can-Fite BioPharma Ltd.
Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CFBI) is an advanced clinical stage drug development Company with a platform technology that is designed to address multi-billion dollar markets in the treatment of cancer, liver, and inflammatory disease. The Company's lead drug candidate, Piclidenoson recently reported topline results in a Phase III trial for psoriasis. Can-Fite's liver drug, Namodenoson, is being evaluated in a Phase IIb trial for the treatment of non-alcoholic steatohepatitis (NASH), and enrollment is expected to commence in a Phase III trial for hepatocellular carcinoma (HCC), the most common form of liver cancer. Namodenoson has been granted Orphan Drug Designation in the U.S. and Europe and Fast Track Designation as a second line treatment for HCC by the U.S. Food and Drug Administration. Namodenoson has also shown proof of concept to potentially treat other cancers including colon, prostate, and melanoma. CF602, the Company's third drug candidate, has shown efficacy in the treatment of erectile dysfunction. These drugs have an excellent safety profile with experience in over 1,500 patients in clinical studies to date. For more information please visit: www.can-fite.com.
Forward-Looking Statements
This press release may contain forward-looking statements, about Can-Fite’s expectations, beliefs or intentions regarding, among other things, its product development efforts, business, financial condition, results of operations, strategies or prospects. All statements in this communication, other than those relating to historical facts, are “forward looking statements”. Forward-looking statements can be identified by the use of forward-looking words such as “believe,” “expect,” “intend,” “plan,” “may,” “should” or “anticipate” or their negatives or other variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical or current matters. Forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to known and unknown risks, uncertainties and other factors that may cause Can-Fite’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Important factors that could cause actual results, performance or achievements to differ materially from those anticipated in these forward-looking statements include, among other things, our history of losses and needs for additional capital to fund our operations and our inability to obtain additional capital on acceptable terms, or at all; uncertainties of cash flows and inability to meet working capital needs; the initiation, timing, progress and results of our preclinical studies, clinical trials and other product candidate development efforts; our ability to advance our product candidates into clinical trials or to successfully complete our preclinical studies or clinical trials; our receipt of regulatory approvals for our product candidates, and the timing of other regulatory filings and approvals; the clinical development, commercialization and market acceptance of our product candidates; our ability to establish and maintain strategic partnerships and other corporate collaborations; the implementation of our business model and strategic plans for our business and product candidates; the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and our ability to operate our business without infringing the intellectual property rights of others; competitive companies, technologies and our industry; risks related to the COVID-19 pandemic and the Russian invasion of Ukraine; risks related to not satisfying the continued listing requirements of NYSE American; and statements as to the impact of the political and security situation in Israel on our business. More information on these risks, uncertainties and other factors is included from time to time in the “Risk Factors” section of Can-Fite’s Annual Report on Form 20-F filed with the SEC on March 24, 2022 and other public reports filed with the SEC and in its periodic filings with the TASE. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Can-Fite undertakes no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by any applicable securities laws.