IRVINE, Calif.--(BUSINESS WIRE)--Endologix LLC, a privately held global medical device company dedicated to improving patients’ lives by providing disruptive therapies for the interventional treatment of vascular disease, announced that the ELEVATE (Expanding Patient Applicability with Polymer Sealing Ovation ALTO Stent Graft) IDE Study has been published online in the Journal of Vascular Surgery. The study reports the clinical outcomes of the ALTO Abdominal Stent Graft System and highlights the device’s safety and effectiveness at one-year post-procedure. Additionally, the results will be presented by the study’s principal investigator, Dr. Sean Lyden, Chairman of the Department of Vascular Surgery at Cleveland Clinic, at the VEITHsymposium in November 2022.
The ELEVATE study enrolled 75 patients between March 2017 and February 2018 in 16 centers in the United States. The study included patients with infrarenal AAAs with neck diameters of 16-30mm at 7mm below the lowest renal artery. Patients were followed for 30 days, 6 months, and 1 year by clinical evaluation, CT, and abdominal x-ray imaging. The study concluded that the Endologix ALTO stent graft is safe and effective in treating AAA with appropriate anatomy at one year. The results presented included:
- Treatment success rate of 96.7% at 12 months
- All-cause mortality of 4.0% at 12 months; no AAA-related mortality occurred
- Major adverse event rate of 5.3% at 30 days
Data from the ELEVATE Study was utilized in a PMA-Supplement to the FDA in 2019. Subsequent approval of the ALTO Abdominal Stent Graft System and commercialization occurred in 2020.
“The publication of results of the ELEVATE Study represent a foundational block of the evidence that we are building for our ALTO product line,” said Matt Thompson, MD, President and CEO of Endologix. “To further expand this evidence base, we have recently begun enrolling patients in the JAGUAR randomized controlled trial, which is a randomized comparative study examining the hypothesis that the ALTO endograft will have superior durability to competitive endografts secondary to a significant reduction in post-EVAR aortic neck dilatation. We believe that ALTO plays a significant role in the management of AAA based on its durability, low-profile, and broadest patient applicability within EVAR.”
Reference
Sean P. Lyden, D. Christopher Metzger, Steve Henao, Sonya Noor, Andrew Barleben, John P. Henretta, Levester Kirksey, One-year safety and effectiveness of the Alto abdominal stent graft in the ELEVATE IDE trial, Journal of Vascular Surgery, (2022), ISSN 0741-5214.
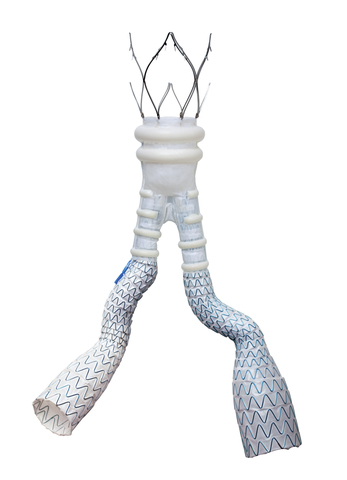
About the ALTO Abdominal Stent Graft System
Featuring a unique, patented, sealing technology, the ALTO Abdominal Stent Graft System is the latest generation of innovative therapies for AAA patients. ALTO utilizes a low-profile delivery system and, unlike standard EVAR devices, features an exclusive Adaptive Sealing Technology that molds in-situ to the patient’s specific aortic neck anatomy.
About Endologix
Endologix LLC is a California-based, global medical device company dedicated to improving patients’ lives by providing innovative therapies for the interventional treatment of vascular disease. Endologix’s therapeutic portfolio includes a variety of products in various stages of development that are designed to treat diseases that currently have clinically relevant unmet needs. These products are designed to treat a wide spectrum of vascular disease from abdominal aortic aneurysms to lower limb peripheral vascular disease. Excellent clinical outcomes will be achieved through meticulous attention to product design, manufacturing, and training, all backed by industry-leading clinical evidence. Endologix’s current commercial EVAR products include the AFX®2 Endovascular AAA System and the ALTO® Abdominal Stent Graft System. On October 1, 2020, Endologix became a private company, wholly owned by Deerfield Management, an investment management firm committed to advancing healthcare through investment, information, and philanthropy. In April 2021, Endologix completed the acquisition of PQ Bypass, Inc., a privately held medical technology, adding the DETOUR System and TORUS Stent Graft to the Company’s product pipeline. The DETOUR System and the TORUS Stent Graft have not been approved for sale by any regulatory body. The DETOUR System is an investigational device, limited by United States law to investigational use.
The company has offices and manufacturing sites in Irvine and Santa Rosa, California. To learn more about Endologix, please visit https://www.endologix.com/.
Except for historical information contained herein, this press release contains forward-looking statements, including statements regarding the enrollment and ultimate success of our current JAGUAR randomized control trial in demonstrating both that the ALTO endograft significantly reduces post-EVAR aortic neck dilatation and that it has superior durability to competitive endografts. Forward-looking statements are subject to risks and uncertainties and other factors that may cause actual results to differ materially from those expressed or implied. The forward-looking statements contained in this press release speak only as of the date of this press release and Endologix undertakes no obligation to update any forward-looking statements contained in this press release to reflect new information, events, or circumstances after the date they are made, or to reflect the occurrence of unanticipated events.