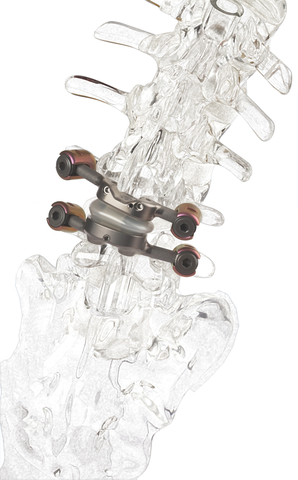
Premia Spine’s TOPS™ Spinal Joint Replacement Shows Potential to Relieve Back Pain and Maintain Mobility Without Adjacent Level Degeneration
Premia Spine’s TOPS™ Spinal Joint Replacement Shows Potential to Relieve Back Pain and Maintain Mobility Without Adjacent Level Degeneration
Case series published in Operative Neurosurgery evaluates long-term data on motion-preserving alternative to fusion
NORWALK, Conn.--(BUSINESS WIRE)--Premia Spine, a medical technology company changing the way debilitating chronic leg and back pain is treated, today announced that a single-center study evaluating its TOPS™ System for lumbar spinal stenosis and degenerative spondylolisthesis has been published in the journal Operative Neurosurgery. In the paper, “Mobility-Maintaining Arthroplasty of the Lumbar Spine with the Second-Generation TOPS System: A Case Series,” authors Werner Lack, M.D., et al., concluded that the TOPS facet replacement has the potential to relieve leg and back pain and maintain close-to-normal range-of-motion (ROM) over four years without inducing adjacent segment disease (ASD). The authors also conclude that the TOPS System is the first to allow patients to settle into their own natural sagittal balance, thus presenting new possibilities for non-fusion treatment of degenerative spondylolisthesis and spinal stenosis with mobility-maintaining dorsal instrumentation.
“The results of our study point to the potential for the TOPS System as a new surgical option for degenerative spondylolisthesis and lumbar spinal stenosis that could relieve leg and back pain while allowing patients to maintain range of motion,” said Dr. Lack. “Outcomes from our experience support a low complication rate with an unusually rapid, and sustained, reduction in pain.”
This study presents a single-center experience with a facet replacement implant designed to stabilize the spine and prevent further degeneration, while maintaining a normal ROM. Seventeen patients who received the implant following a laminotomy were evaluated for long-term improvements in visual analog scale (VAS) and ROM over an average of 51 months (range of 26-77). The average reduction in VAS scores was 7.5 at 3 months, 6.8 at 12 months, and 6.7 at the longest follow up, demonstrating an average improvement of 81%. The preoperative and post-operative average ROMs at the index level were 8.2 degrees and 7.4 degrees, respectively.
“We are encouraged by these long-term results, which support other studies that demonstrate the potential of the TOPS System as a viable alternative to fusion for the treatment of spinal stenosis and degenerative spondylolisthesis,” said Ron Sacher, CEO, Premia Spine. “Our goal remains to deliver an implant that allows patients to retain the natural motion of their spine with less pain. These data bolster our case for TOPS as a new treatment paradigm for patients suffering from these diseases.”
The first and only facet joint replacement system for the lumbar spine, TOPS was developed to provide mobility, stability and durability after decompression for patients with lumbar spinal stenosis and degenerative spondylolisthesis. Spinal stenosis and degenerative spondylolisthesis are painful, debilitating and highly prevalent conditions impacting over 100 million people globally1. An estimated 350,000 people undergo lumbar spinal fusion each year for these conditions2, representing a $2 billion annual addressable global market.
TOPS won Breakthrough Device Designation from the US Food and Drug Administration (FDA) in 2021 and is currently the subject of a pivotal clinical trial under an investigational device exemption from the FDA.
About Premia Spine
Premia Spine, a medical technology company, aims to improve the lives of chronic leg and back pain patients with its TOPS™ System. TOPS is designed to provide lasting mobility, stability and durability to patients with lumbar spinal stenosis, degenerative spondylolisthesis and related spinal conditions.
1 Ravindra, V. M., et al. Degenerative Lumbar Spine Disease: Estimating Global Incidence and Worldwide Volume. Global Spine Journal: 2018, Vol 8, Issue 8. https://journals.sagepub.com/doi/full/10.1177/2192568218770769
2 2019 Spinal Surgery Update. Orthopedic Network News: Volume 30, Number 4, October 2019.
Contacts
Joe Duraes
Pazanga Health Communications
jduraes@pazangahealth.com
(917) 687-6419