BURLINGAME, Calif.--(BUSINESS WIRE)--Magnus Medical, Inc., a medical device company and developer of brain stimulation technology for treatment of neuropsychiatric disorders, today announced it received 510(k) clearance from the U.S. Food & Drug Administration (FDA) for the SAINTTM Neuromodulation System for the treatment of major depressive disorder (MDD) in adults who have failed to achieve satisfactory improvement from prior antidepressant medications in the current episode.
“Magnus’ SAINT technology is groundbreaking and could help many patients with major depressive disorder (MDD) who have not responded to treatment with antidepressants,” said Alan F. Schatzberg, M.D., the Kenneth T. Norris, Jr. Professor of Psychiatry and Behavioral Sciences at Stanford University, and a Past President of the American Psychiatric Association. ”The treatment effects observed with SAINT treatment in the double-blinded, randomized controlled trial that was published in the American Journal of Psychiatry were dramatic, rapid, and frequently sustained through the study follow-up period. The technology could result in a fundamental change in the treatment approach to patients with refractory MDD and has the potential to reduce both the morbidity and mortality associated with the disorder.”
“This FDA clearance of the SAINT Neuromodulation System for depression is really exciting news,” said Mark S. George, M.D., distinguished professor of Psychiatry, Radiology and Neuroscience, and director of the Brain Stimulation Division, Psychiatry at the Medical University of South Carolina. “This is more than just clearance of another device. This clearance expands the way we can use TMS to treat depression. The older approaches often took six weeks for depression to respond, while this approach observed remission from depression in just five days. That opens up many new possibilities to use SAINT in hospitalized patients, for patients who present to the emergency room, and with different schedules in clinics.’'
The American Journal of Psychiatry published results from a double-blinded randomized controlled trial (RCT) evaluating SAINT that shows 79% of people in the active treatment arm entered remission from their depression compared to 13% in the sham treatment arm.
The SAINT Neuromodulation System, which received Breakthrough Device Designation from the FDA, a program intended to help patients receive more timely access to breakthrough technologies with the potential to provide more effective treatment for life-threatening or debilitating conditions than previous therapies, is a novel innovation that is significantly impacting the treatment of severe depression. For the first time, advanced imaging technologies combined with personalized targeting and novel stimulation patterns have yielded a new form of individualized neurostimulation for people with treatment-resistant depression.
“We are now at the forefront of an enormous improvement in the care of treatment-resistant depression, thanks to the work of the Magnus team and all those whose efforts have led to the SAINT technology. Today’s FDA’s clearance for the SAINT Neuromodulation System is a major milestone in our long-term journey to restore and sustain mental health,” said Brett Wingeier, Ph.D., co-founder and CEO of Magnus. “More broadly, we look forward to seeing this work make a positive impact for the millions of people affected by neuropsychiatric disease. Our clinical research program will continue to yield more insights into how personalized neuromodulation can restore healthy neural activity across a wide variety of mental health conditions.
“We expect the commercial launch of our SAINT Neuromodulation System to begin later in 2023 on a limited basis, for which we are building a waitlist and engaging with an overwhelming number of teaching institutions, hospitals, clinics, interested clinicians, and medical professionals,” continued Dr. Wingeier.
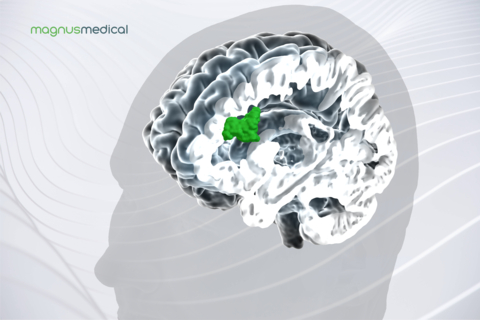
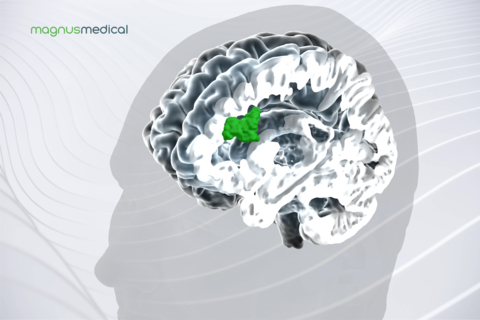
The SAINT Neuromodulation System uses structural and functional magnetic resonance imaging (MRI) to inform a proprietary algorithm that identifies the optimal anatomic target for focused neurostimulation in people with MDD. This new approach is delivered on an accelerated timeline and is precisely tailored to each person’s brain connectivity.
About Magnus
Magnus Medical, Inc., is a privately held medical device company driven to transform lives by restoring mental health. Magnus’ first FDA-cleared product is the SAINT Neuromodulation System, which received FDA Breakthrough Device Designation for its novel, rapid-acting therapy for treatment-resistant depression. The SAINT Neuromodulation System yields a new form of individualized neurostimulation for adults who have failed to achieve satisfactory improvement from prior antidepressant medications. Magnus’ leadership team includes Brett Wingeier, Ph.D., chief executive officer, Brandon Bentzley, M.D., Ph.D., chief scientific officer, and Scott Ashworth, chief commercial officer. For more information, visit https://www.magnusmed.com.