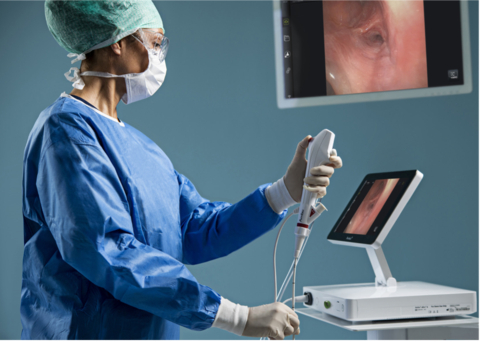
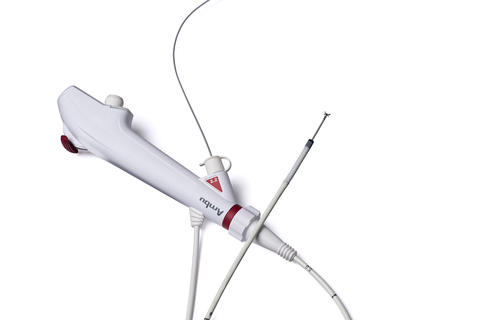
COLUMBIA, Md.--(BUSINESS WIRE)--Ambu Inc. announces that Ambu® aScopeTM 5 Broncho, a family of single-use, sterile bronchoscopes, has received 510(k) regulatory clearance from the U.S. Food and Drug Administration (FDA).
Ambu announced European regulatory clearance in May 2022 and will now proceed with commercialization of the aScope 5 Broncho and the full high definition Ambu® aBoxTM 2 processing unit in Europe as well as in the USA.
ADVANCED DESIGN FEATURES AND SUPERIOR IMAGE QUALITY
With the aScope 5 Broncho, Ambu now leads the entry of single-use endoscopes in the bronchoscopy suite, a market segment known for its considerably complex medical procedures — procedures that require scopes of high-performance image quality and handling. To enter this market, the aScope 5 Broncho family has advanced imaging and design features, including a new high-resolution camera chip, which, in combination with the aBox 2, delivers superior image quality.
“With the aScope 5 Broncho system, I am getting a complete package with all the things that I need for it to be my workhorse scope in the bronchoscopy suite. The way I see where the field is going, I think this is a bronchoscope of the future,” said Dr. Ashutosh Sachdeva1 , Director of Interventional Pulmonology Program at University of Maryland Medical Center in the Division of Pulmonary and Critical Care, and Assistant Professor of Medicine at the University of Maryland School of Medicine
For healthcare professionals and patients alike in the bronchoscopy suite, Ambu’s fifth-generation bronchoscope offers sterility as well as a consistently high performance, placing patient safety at the heart of every procedure. Furthermore, the advanced technology, portability, and cost-effectiveness of Ambu’s solution makes it an attractive choice for health care providers who need to perform bronchoscopies — not only in the bronchoscopy suite, but across a wide range of care settings, such as operating rooms, intensive care units, and emergency rooms.
EXTENDING MARKET LEADERSHIP IN PULMONOLOGY
The launch of the aScope 5 Broncho family follows Ambu’s ambition of establishing the most comprehensive single-use visualization portfolio within pulmonology.
“We introduced the world’s first flexible single-use bronchoscope 13 years ago, breaking new ground in intensive care units and operating rooms worldwide. However, until now, the advanced needs in the bronchoscopy suite were never met by single-use scopes. Now, we have created a single-use portfolio that meets these needs – one that is on par with reusable bronchoscopes, in some areas even superior,” said Bassel Rifai, Chief Marketing Officer at Ambu. “
1 Dr. Sachdeva is a paid consultant of Ambu A/S. He has not been compensated for his quote in this press release.
ABOUT AMBU
Ambu has brought solutions of the future to life since 1937. Today, millions of patients and healthcare professionals worldwide depend on the efficiency, safety and performance of our single-use endoscopy, anesthesia and patient monitoring solutions. We continuously look ahead with a commitment to deliver innovative quality products that have a positive impact on patient care and the work of healthcare professionals. Headquartered near Copenhagen in Denmark, Ambu employs approximately 5,000 people in Europe, North America and Asia-Pacific. For more information, please visit Ambu.com or AmbuUSA.com or follow us on our Corporate LinkedIn and USA LinkedIn pages.