BALTIMORE--(BUSINESS WIRE)--ReGelTec, Inc., announced that Dr. Douglas Beall won the award for Best Abstract at the American Society for Pain and Neuroscience annual meeting that just concluded in Miami, FL. Dr. Beall presented an interim analysis of clinical data from feasibility studies of the company’s HYDRAFIL™ technology for the treatment chronic low back pain (CLBP) that included 3-month follow-up results on sixty patients.
Baltimore based ReGelTec is developing a percutaneous treatment for CLBP caused by degenerative disc disease (DDD), a widely prevalent condition affecting an estimated 9% of the worldwide population and costing upwards of $100B in direct treatments and lost productivity in the United States alone.
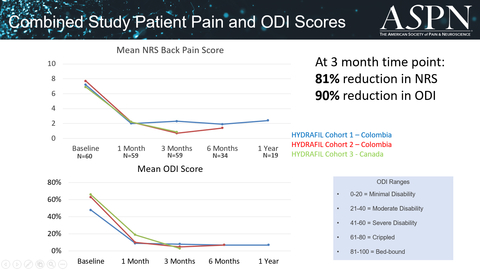
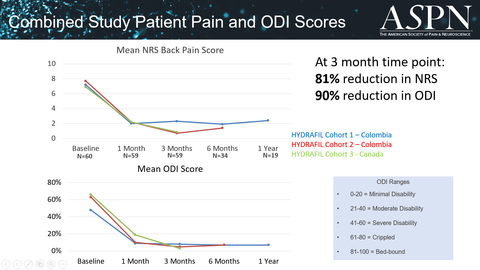
To date, the company has conducted clinical studies in Barranquilla and Cali, Colombia, and in Calgary, Canada with 60 patients receiving HYDRAFIL™ injections. Patients have experienced profound relief in their back pain and related disability scores. At the three month follow up visit the average back pain score is reduced by 81%, from a baseline of 7.3 to a score of 1.4, and disability scores measured by Oswestry Disability Index (ODI) dropped by 90%, from a baseline of 58% to a score of 6%.
“The results we are seeing with HYDRAFIL to date are nothing short of astounding,” said Douglas Beall, MD Interventional Radiologist and Chief of Radiology Services at Clinical Radiology of Oklahoma. “I’m excited to continue to generate long term clinical data to show the impact that HYDRAFIL can have on chronic low back pain because I see patients in my practice every week who will be candidates for this therapy.”
The 60 patients in clinical studies to date were successfully treated with HYDRAFIL, a patented hydrogel that is melted prior to injection into the nucleus of a degenerated disc via a needle. When HYDRAFIL cools to body temperature it forms a contiguous implant within the nucleus of the degenerated disc to augment the residual nucleus pulposus, restore normal biomechanical properties of the disc and alleviate pain. The procedures were completed while patients were awake and under local anesthesia in an outpatient clinic. Patients were up and walking within one to two hours of the injection. Most patients were sent home on standard over the counter pain medication and procedural related pain generally resolved within 24-72 hours. Patient follow-up is ongoing and will be continued out to at least a year for all study participants.
Bill Niland, a serial entrepreneur and the company’s CEO said “We are grateful to Dr. Beall, the study investigators, and the American Society for Pain and Neuroscience for their support and recognition as we continue to advance HYDRAFIL. We know that it will be a significant addition to the treatments available for chronic low back pain outside of the standard conservative care or surgical treatment options. The company is excited to expand our clinical studies further and generate additional evidence showing both the clinical impact and cost effectiveness of HYDRAFIL to help the millions of people with chronic low back pain.”
ABOUT REGELTEC, INC:
ReGelTec, Inc. is a clinical stage medical device company commercializing HYDRAFIL™, a percutaneous treatment for low back pain due to degenerative disc disease. The company was formed when a team of chemical engineers with extensive experience in polymer science partnered with a cross-functional team of medical device professionals with multiple successful startup exits. Once approved, the HYDRAFIL™ System will offer patients suffering from chronic back pain due to degenerative disc disease a minimally invasive treatment option beyond traditional conservative care. HYDRAFIL is an investigational device, limited by United States law to investigational use.