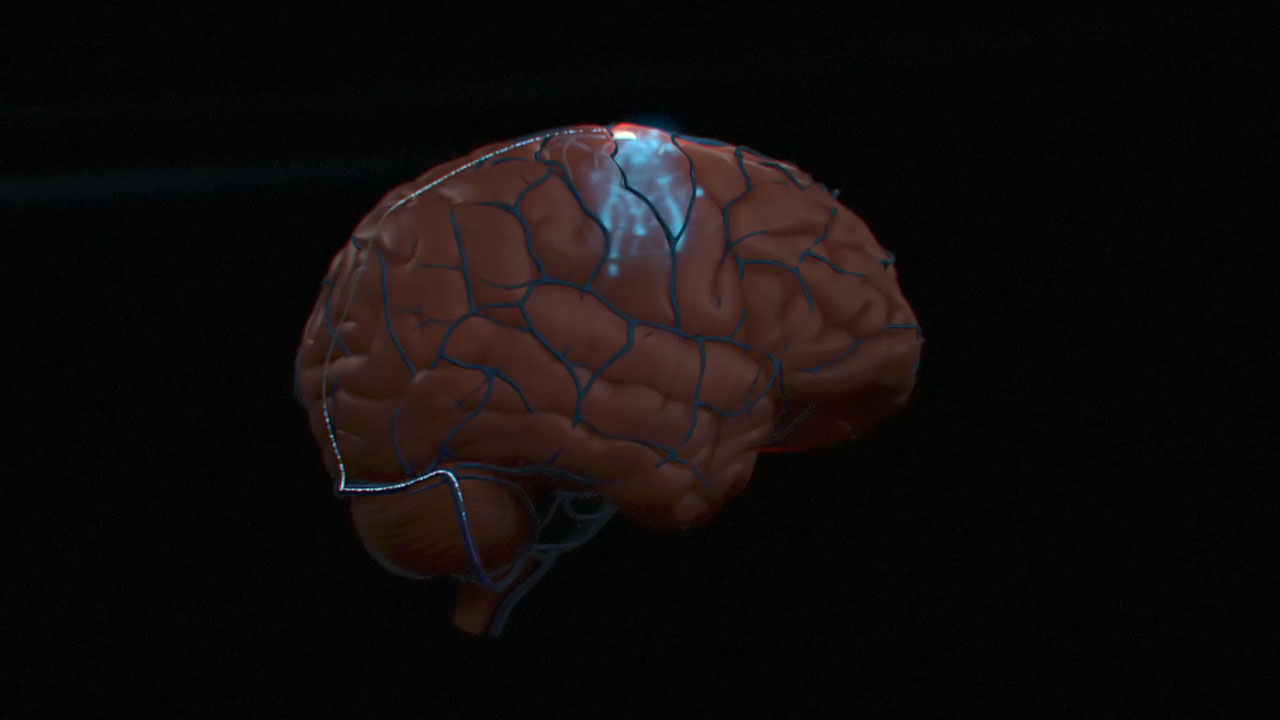
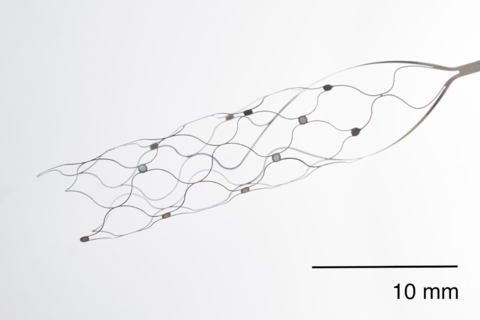
NEW YORK--(BUSINESS WIRE)--Synchron, an endovascular brain-computer interface (BCI) company, today announced the first human BCI implant in the United States. This procedure represents a significant technological milestone for scalable BCI devices and is the first to occur in the U.S. using an endovascular BCI approach, which does not require invasive open-brain surgery.
The procedure was performed at Mount Sinai West in New York, led by clinical investigator Shahram Majidi, MD, assistant professor of neurosurgery, neurology and radiology at the Icahn School of Medicine at Mount Sinai. The procedure was performed in the angiography suite with a minimally invasive, endovascular approach. Mount Sinai’s Department of Rehabilitation and Human Performance helped coordinate the procedure.
The procedure marks the first U.S. patient implant in Synchron’s COMMAND trial, which is being conducted under the first investigational device exemption (IDE) awarded by the FDA to a company assessing a permanently implanted BCI. The U.S.-based trial is being conducted with support from the NIH Neural Interfaces Program.
The COMMAND study will assess the safety and efficacy of the company’s motor BCI technology platform, including the Stentrode™, in patients with severe paralysis with the goal of enabling the patient to control digital devices hands-free. Study outcomes include the use of brain data to control digital devices and achieve improvements in functional independence.
“This is an incredibly exciting milestone for the field, because of its implications and huge potential,” said Shahram Majidi, MD, the neurointerventional surgeon who performed the procedure, and assistant professor of neurosurgery, neurology and radiology at the Icahn School of Medicine at Mount Sinai. “The implantation procedure went extremely well, and the patient was able to go home 48 hours after the surgery."
“We are beyond excited to get to work with our patient, guiding them through the training process as they learn to use this device to live more independently and, most importantly, communicate with their family and friends,” said David Putrino, PhD, PT, Director of Rehabilitation Innovation for the Mount Sinai Health System and a Principal Investigator of the COMMAND study.
“The first-in-human implant of an endovascular BCI in the U.S. is a major clinical milestone that opens up new possibilities for patients with paralysis,” said Tom Oxley, MD, PhD, CEO & Founder, Synchron. “Our technology is for the millions of people who have lost the ability to use their hands to control digital devices. We’re excited to advance a scalable BCI solution to market, one that has the potential to transform so many lives.”
The Stentrode is implanted within the motor cortex of the brain via the jugular vein in a minimally-invasive endovascular procedure. Once implanted, it detects and wirelessly transmits motor intent using a proprietary digital language to allow severely paralyzed patients to control personal devices with hands-free point-and-click. The trial will assess the impact of everyday tasks such as texting, emailing, online shopping and accessing telehealth services, and the ability to live independently. The FDA granted Breakthrough Device designation to Synchron in August 2020.
Synchron will continue to advance enrollment in its COMMAND trial as the industry-first FDA-approved clinical trial for a permanently implanted BCI in the U.S. Recently reported long-term safety results have demonstrated this technology to be safe in four patients out to 12 months in Synchron’s SWITCH trial in Australia, as reported at the 2022 American Academy of Neurology Conference.
About the Stentrode™
Synchron’s flagship technology, the Stentrode, is an endovascular brain implant designed to enable patients to wirelessly control digital devices through thought and improve functional independence. Synchron’s foundational technology, a motor neuroprosthesis (MNP), or motor BCI, is implanted via the jugular vein using neurointerventional techniques commonly used to treat stroke, and does not require drilling into the skull or open-brain surgery. The system is designed for patients suffering from paralysis as a result of a range of conditions. It is designed to be user friendly and dependable for patients to use autonomously.
About Synchron, Inc.
Synchron, an endovascular brain interface company, is a leader in implantable neural interface technology. The clinical-stage company is developing a neuroprosthesis for the treatment of paralysis and the first endovascular implantable neuromodulation therapy. Future applications include the potential to diagnose and treat conditions of the nervous system, including Parkinson’s disease, epilepsy, depression, and hypertension. Synchron is headquartered in New York City, with R&D facilities in Melbourne, Australia. For more information, visit www.synchron.com. Follow us on Twitter @synchroninc.