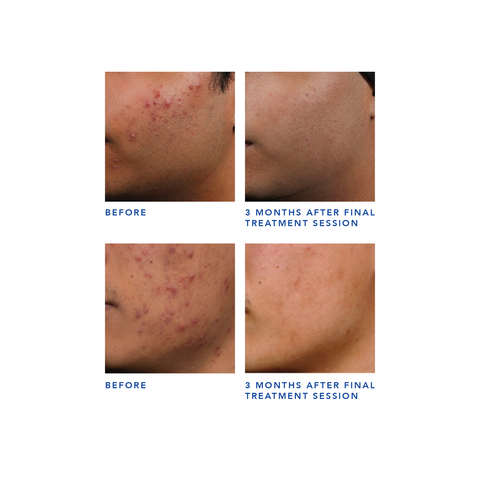
TORONTO--(BUSINESS WIRE)--Cutera, Inc. (Nasdaq: CUTR), a leading provider of aesthetic and dermatology solutions, today announced that AviClear, the first and only energy device approved in the United States for the treatment of mild, moderate, and severe acne, has secured clearance from Health Canada for the treatment of mild, moderate, and severe acne and acne scars. This Health Canada announcement comes on the heels of the U.S. Food and Drug Administration’s 510(k) clearance of AviClear, in March 2022.
AviClear is a laser treatment that offers a safe, effective, prescription-free solution for acne. In addition to reducing existing acne, clinical trials show that future breakout episodes are shorter, less intense, and more infrequent following three 30-minute treatment sessions. Further, acne clearance results continue to improve over time, demonstrating the long-term efficacy of this novel treatment. Importantly, no pain mitigation was utilized or required by any clinical study participant.1
Acne vulgaris is a nearly universal skin disease, with approximately 50 million North American teens and young adults seeking treatment each year.2 AviClear’s 1726nm wavelength treats acne at the source by selectively targeting the sebocytes and suppressing sebum production. Overproduction of sebum by the sebaceous glands is one of the leading causes of acne.3
“Acne affects so many people – from teens to women in menopause, and everyone in between,” said Jerry K. L. Tan, MD, FRCPC. “I am thrilled that AviClear can offer patients a safe, effective, and drug-free treatment option that will help them face the world without the worry, frustration or self-consciousness that often accompanies acne.”
“We are ecstatic with AviClear’s expansion into the Canadian market as an innovative tool for dermatologists and as a treatment that inspires hope for acne patients,” said David Mowry, CEO of Cutera. "Developed with extensive physician and patient input, AviClear is helping to redefine and optimize modern acne care.”
Canadian doctors and consumers are encouraged to visit www.AviClear.com and sign up for updates on product availability and local treatment providers. Cutera is planning for measured expansion of AviClear to Canadian providers in their limited commercial release over the course of 2022.
About Cutera, Inc.
Brisbane, California-based Cutera is a leading provider of aesthetic and dermatology solutions for practitioners worldwide. Since 1998, Cutera has been developing innovative, easy-to-use products that harness the power of science and nature to enable medical practitioners to offer safe and effective treatments to their patients. For more information, call +1 415-657-5500 or 1-888-4CUTERA or visit www.cutera.com.
References
- Data on file, FDA clearance study. Cutera, Inc.
- https://jamanetwork.com/journals/jamadermatology/fullarticle/479093
- O’Neill AM, Gallo RL. Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome 2018;6: 177.