BELLEVUE, Wash.--(BUSINESS WIRE)--CurvaFix, Inc., a developer of medical devices to repair fractures in curved bones, announced today that it will showcase the new CurvaFix® IM Implant at the American Academy of Orthopaedic Surgeon’s (AAOS) 2022 Annual Meeting in Chicago. In addition to showcasing the novel CurvaFix Implant in its booth (#3669), the company will provide updates from recent U.S. cases using the CurvaFix Implant for fixation of pelvic and acetabular fractures.
“The patients we have treated using the new CurvaFix Implant have tolerated their surgeries well,” said Samir Mehta, M.D., chief of the Division of Orthopaedic Trauma and Fracture Care and associate professor in Orthopaedic Surgery. “The CurvaFix Implant has tremendous versatility allowing surgeons to follow the natural shape of the bone, filling the cancellous space, and achieving a stable construct. Most recently, we treated a 70-year-old female metastatic cancer patient with a bilateral sacral fragility fracture resulting in chronic pain and disability. We were able to provide effective fixation of her dysmorphic upper sacral segment using two CurvaFix Implants placed trans-iliac trans-sacral with the patient being able to walk out of the hospital the same day.”
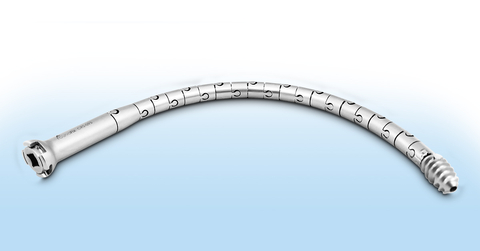
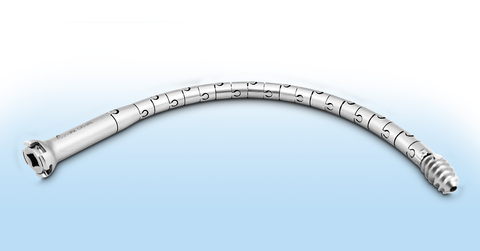
The CurvaFix Implant is the only implant capable of following natural bone curvature to fill space within the bone, designed to offer a less invasive, shorter surgery for pelvic fracture patients with a quicker recovery. The implant body has interlocking segments that provide flexibility during implantation over a steerable guidewire. After implantation, the surgeon locks the CurvaFix Implant curvature (making the device rigid) by setting the patented Lock.
“Our novel device has been used in multiple market segments including high impact trauma, fragility fractures in geriatric patients, revision surgeries for failed straight screw fixation, and in an oncology patient who suffered a fragility fracture due to cancer treatment,” said Steve Dimmer, chief executive officer. “The results thus far are very encouraging, as is our early clinical data.
“The CurvaFix Implant is the only intramedullary device capable of following the natural bone shape and filling the space within the pelvis, enabling a longer, wider and curved implant which provides strong fracture fixation,” continued Dimmer. “The strong curved fixation of our device may improve patient recovery, reduce pain and enable earlier mobility compared to traditional surgical techniques.”
Pelvic fractures affect more than 165,000 people per year in the U.S,1 caused by car accidents or falls. These fractures are among the most serious and technically complex injuries treated by orthopedic trauma surgeons. Current surgical techniques with straight screws can be limited by bone curvature, and surgery with bone plates can require lengthy, invasive open procedures. Pelvic fragility fractures affect nearly 100,000 people annually in the U.S.2 Pelvic fragility fracture repair can be limited by patient health, weak bone and bone curvature.
About CurvaFix, Inc.
CurvaFix, Inc. is a privately held medical device company headquartered in Bellevue, Wash. The idea for the company germinated when the former division head of orthopedic trauma at the University of British Columbia, Clinical Professor Emeritus, Robert Meek, M.D., FRCSC, believed there was a better way to repair pelvic fractures. Today, CurvaFix is developing implantable products to improve fracture repair in curved bones. The company’s first product is the CurvaFix IM Implant, which has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
CurvaFix is a trademark of CurvaFix, Inc., registered in the U.S.
12021 Trauma Update, Orthopedic Network News, Volume 32, No. 2, April 2021
2Orthopedic News Network (ONN), Vol 32, No 2, April 2021