MADISON, Wis.--(BUSINESS WIRE)--New research by Usona Institute identifies the most favorable environments to achieve consistency and reproducibility of pharmaceutical psilocybin produced at large scale. The study, published today in ACS Omega, describes that the ability of a unique crystalline arrangement to solidify in more than one form, called polymorphs, can be optimized using precise combinations of engineering controls including temperature, cooling rate, saturation, stirring speed, and drying mode. Applying these findings, the paper details the formation of three psilocybin polymorphs: polymorph B (trihydrate), polymorph A (anhydrate), and polymorph H (anhydrate).
“Usona has always been interested in studying the intrinsic behavior of naturally occurring molecules, which can be daunting but provide the insights necessary in creating a very reproducible, and high-quality material,” says Robert Kargbo, Medicinal Chemist at Usona Institute, and lead author on the paper. “The more we understand each mechanism and change, the more efficient we become in generating the same quality of material every time.”
Controlled crystallization process
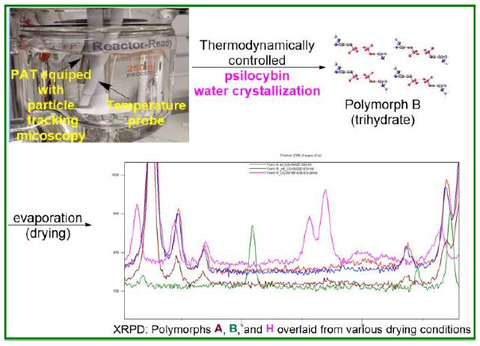
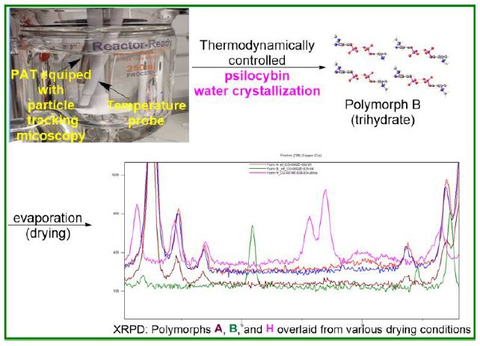
Usona chemists designed a thermodynamically controlled crystal engineering process using measurement of the metastable zone width (MSZW) and nucleation induction to produce a unique crystalized form of psilocybin with stronger interactions, controlled particle size distribution (PSD), and improved impurity profile. A high-resolution inline microscopy viewer allowed for real-time monitoring of the crystallization process and measurement of particle size distribution.
Polymorphs B, A, and H
Researchers applied this method to study polymorph B (trihydrate), polymorph A (anhydrate), and polymorph H (anhydrate) using water recrystallization. Findings showed that formation of polymorph B (trihydrate) is independent of the water-crystallization method used. However, polymorphs A and H are dependent on the mode of drying. Drying at room temperature under vacuum results in mainly polymorph A, and when heated even at slightly above ambient temperatures, a mixture of polymorphs A and H begins to form.
The study also identified the systematic evolution of engineering controls that yield pure anhydrous polymorph A as a single reproducible polymorph at a large scale. The process required controlled drying time and the thin spread of active pharmaceutical ingredient (API) during the drying process. Researchers point out that this work showcases the importance of studying the peculiar impact of drying on polymorph formation.
Provided as open access
Appreciating that these structures have been explored and synthesized for decades, the study published today in ACS Omega is supported through Usona as an open access paper.
Psilocybin is a naturally occurring substance found in numerous species of mushrooms known for their psychoactive properties. Following its chemical synthesis in 1959, purified synthetic psilocybin has been evaluated in clinical trials aimed at understanding its efficacy in treating a range of mental health conditions including addiction, depression, and anxiety.
To learn more about Usona, visit www.UsonaInstitute.org
About Usona Institute
Usona Institute is a 501(c)(3) nonprofit medical research organization (MRO) that conducts and supports pre-clinical and clinical research to further the understanding of the therapeutic effects of psilocybin and other consciousness-expanding medicines. Its focus is on alleviating depression and anxiety in people for whom current medical treatments fall short in offering relief and a better quality of life.