LOS ANGELES--(BUSINESS WIRE)--Sensydia, an innovator in rapid, non-invasive measurement of critical cardiac function, today announced that its Cardiac Performance System (CPSTM) has been granted Breakthrough Device Designation by the United States Food and Drug Administration (FDA). As part of this Breakthrough Device Designation, the FDA will work closely with Sensydia to expedite the development of CPS and prioritize the review of subsequent regulatory submissions.
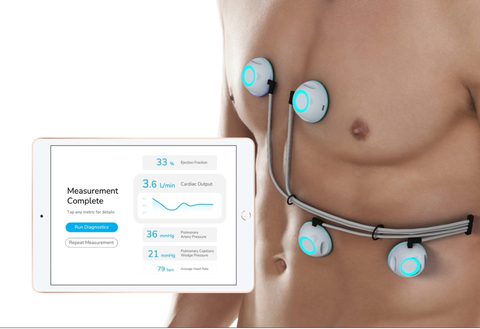
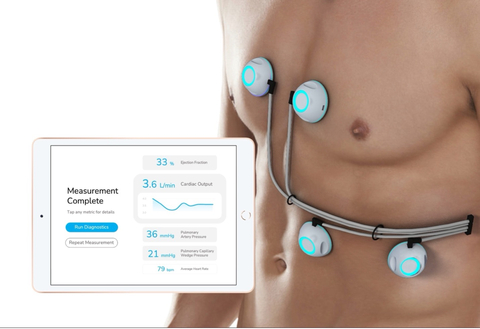
CPS is a non-invasive cardiac monitoring device that reports critical heart performance measurements to physicians for comprehensive evaluation of patients with advanced and persistent heart failure, without requiring an in-hospital catheterization procedure or ultrasound assessment.
Through its ultra-sensitive biosensors, CPS acquires heart sound data and applies machine learning to compute multiple hemodynamic measurements, delivering all in one comprehensive report via the CPS iPad app. CPS can currently measure ejection fraction (already FDA cleared), cardiac output, pulmonary artery pressure, and pulmonary capillary wedge pressure simultaneously.
“Sensydia’s acoustic sensing technology and advanced AI algorithms form the basis of CPS, and together with our stellar research and development capabilities, position it as a breakthrough device,” said Anthony Arnold, President & CEO of Sensydia. “This breakthrough designation is a significant milestone for Sensydia. We look forward to working with the FDA to advance the development of CPS and bring it to market in 2023.”
“The approach taken by the Sensydia team towards cardiac care is impressive and second to none,” said Victoria Grace, Founder of Colle Capital and Sensydia Board Member. “We believe this special FDA designation will accelerate our ability to bring this transformational device to market and make a positive impact on the lives of patients.”
Heart failure is a devastating and debilitating public health epidemic that affects more than 6 million Americans and costs the nation $30.7 billion annually. Hemodynamic measurements, which describe how well the heart is functioning, are essential in the diagnosis and management of patients with heart disease who are at a high level of clinical risk. However, obtaining these measurements currently requires specialized techniques such as echocardiography and invasive in-hospital cardiac catheterization, which can cause significant delays in treatment. Sensydia CPS is designed to provide accurate, comprehensive, and non-invasive hemodynamic assessment within minutes without the need for a highly skilled operator in a range of healthcare settings.
With its Cardiac Performance System (CPSTM), Sensydia is developing a new cardiac monitoring technology that enables more effective management of patients anywhere in the continuum of the heart failure spectrum without the need for in-hospital procedures or multiple diagnostic tests. In 2018, Sensydia received FDA 510(k) clearance for non-invasive measurement of ejection fraction by CPS. Sensydia is on a mission to improve the quality of human life by democratizing non-invasive health assessment and is committed to serving the needs of patients and healthcare providers by delivering high-quality, non-invasive, and easy-to-use products. For more information, visit www.sensydia.com.