Six-Year Natural History Comparison with Mirum’s LIVMARLI (maralixibat) Demonstrates Event-Free Survival and Transplant-Free Survival in Patients with Alagille Syndrome
Six-Year Natural History Comparison with Mirum’s LIVMARLI (maralixibat) Demonstrates Event-Free Survival and Transplant-Free Survival in Patients with Alagille Syndrome
- Results demonstrate statistically significant improvement in six-year event-free survival (p<0.0001) and transplant-free survival (p<0.0001)
- Study presented by Global Alagille Alliance (GALA) at The Liver Meeting® (AASLD) in a late-breaking oral presentation; selected to be featured in Best of Liver Meeting series
- Data underscore LIVMARLI’s potential to delay liver transplant and improve disease outcomes associated with Alagille syndrome
FOSTER CITY, Calif.--(BUSINESS WIRE)--Mirum Pharmaceuticals, Inc. (Nasdaq: MIRM) today announced that a new analysis comparing pooled LIVMARLI™ (maralixibat) oral solution clinical trial data with a natural history cohort was presented at the American Association for the Study of Liver Diseases annual congress, The Liver Meeting®. LIVMARLI is an oral ileal bile acid transporter (IBAT) inhibitor recently approved by the U.S. Food and Drug Administration for the treatment of cholestatic pruritus in patients with Alagille syndrome (ALGS) one year of age and older.
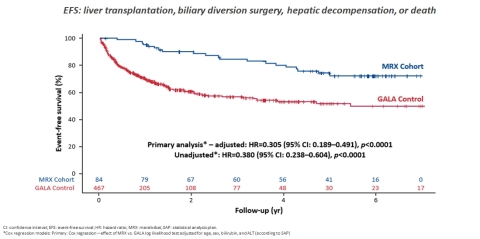
The oral, late-breaking session, which was selected as part of the “Best of the Liver Meeting” series, reported on an analysis independently conducted and presented by Dr. Bettina Hansen on behalf of Dr. Binita Kamath and the Global Alagille Alliance (GALA) Study Group, which has aggregated the largest global natural history clinical database established for ALGS. The analysis evaluated six years of follow-up from pooled LIVMARLI studies (n=84) in ALGS and compared it against an external natural history control cohort from the GALA database. The objective of the analysis was to compare the time to first clinical event in LIVMARLI-treated patients with ALGS versus a natural history cohort, with events defined as liver transplantation, biliary diversion surgery, decompensation events (ascites requiring therapy or variceal bleeding), or death. Additional analyses included transplant-free survival as well as several sensitivity and subgroup analyses to ensure robustness of the findings. The GALA control group was selected based on alignment with eligibility criteria from the LIVMARLI clinical studies.
The analysis demonstrated a highly significant improvement in six-year event-free survival with a p-value of <0.0001 (HR: 0.305, 95% CI: 0.189-0.491) translating to a 70% overall reduction for clinical outcomes with LIVMARLI. The analysis also showed statistically significant improvements in transplant-free survival with a p-value of <0.0001 (HR: 0.332, 95% CI: 0.197-0.559). These data underscore LIVMARLI’s potential to have long-term and sustained impact for patients with ALGS.
“The extensive real-world analysis conducted by GALA validates the statistical robustness of the findings and suggest that patients who are treated with LIVMARLI may experience statistically significant event-free survival and transplant-free survival versus the natural history cohort,” said Bettina Hansen, Ph.D., Associate Professor of Biostatistics at the Toronto Centre for Liver Disease, Toronto General Hospital, Toronto General Hospital & University of Toronto, Canada, on behalf of the GALA Study Group.
“Cholestatic pruritus associated with Alagille syndrome has a dramatic and debilitating impact on the lives of patients and is a leading indication for liver transplantation,” said Pam Vig, Ph.D., Chief Scientific Officer and Head of R&D at Mirum. “The six-year analysis demonstrates that LIVMARLI significantly improves event-free survival and transplant-free survival, further supporting the impact of LIVMARLI in this patient population.”
Also at the meeting, a second late-breaker presentation was given by Dr. Ron Sokol. These complementary data analyzed predictors of event-free survival and transplant-free survival in 76 patients treated with LIVMARLI. Variables that were predictive of event-free survival included specific thresholds of week 48 total bilirubin, and week 48 serum bile acids. Change in baseline to week 48 in pruritus was also predictive of event-free survival and transplant-free survival. Sixty out of 76 patients remained event-free at the time of analysis, with up to six years of treatment with LIVMARLI. These data highlighted potential prognostic markers that could help to inform medical management for patients receiving LIVMARLI treatment.
To learn more about the data presented during the meeting, please visit the Alagille syndrome section of our Publications & Presentations page on Mirum’s website.
The results of the natural history comparison analysis were included in Mirum’s recent marketing authorization application submission (MAA) to the European Medicines Agency (EMA) for LIVMARLI in the treatment of cholestatic liver disease in patients with ALGS. The MAA is currently under review and Mirum is preparing for a launch of LIVMARLI in Europe in the second half of 2022, if approved by the EMA.
About LIVMARLI™ (maralixibat) oral solution
LIVMARLI™ (maralixibat) oral solution is an orally administered, once-daily, ileal bile acid transporter (IBAT) inhibitor approved by the U.S. Food and Drug Administration for the treatment of cholestatic pruritus in patients with Alagille syndrome one year of age and older and is the only FDA-approved medication to treat cholestatic pruritus associated with Alagille syndrome. For more information, please visit LIVMARLI.com.
LIVMARLI is currently being evaluated in late-stage clinical studies in other rare cholestatic liver diseases including progressive familial intrahepatic cholestasis and biliary atresia. LIVMARLI has received Breakthrough Therapy designation for ALGS and PFIC type 2 and orphan designation for ALGS, PFIC and biliary atresia. To learn more about ongoing clinical trials with LIVMARLI, please visit Mirum’s clinical trials section on the company’s website.
About Alagille syndrome
Alagille syndrome (ALGS) is a rare genetic disorder in which bile ducts are abnormally narrow, malformed and reduced in number, which leads to bile accumulation in the liver and ultimately progressive liver disease. The estimated incidence of ALGS is one in every 30,000 people.1 In patients with ALGS, multiple organ systems may be affected by the mutation, including the liver, heart, kidneys and central nervous system.2 The accumulation of bile acids prevents the liver from working properly to eliminate waste from the bloodstream and, according to recent reports, 60% to 75% of patients with ALGS have a liver transplant before reaching adulthood.3 Signs and symptoms arising from liver damage in ALGS may include jaundice (yellowing of the skin), xanthomas (disfiguring cholesterol deposits under the skin), and pruritus (itch)2. The pruritus experienced by patients with ALGS is among the most severe in any chronic liver disease and is present in most affected children by the third year of life.4
IMPORTANT SAFETY INFORMATION
LIVMARLI can cause serious side effects, including:
Changes in liver tests. Changes in certain liver tests are common in patients with Alagille syndrome and can worsen during treatment with LIVMARLI. These changes may be a sign of liver injury and can be serious. Your healthcare provider should do blood tests before starting and during treatment to check your liver function. Tell your healthcare provider right away if you get any signs or symptoms of liver problems, including nausea or vomiting, skin or the white part of the eye turns yellow, dark or brown urine, pain on the right side of the stomach (abdomen) or loss of appetite.
Stomach and intestinal (gastrointestinal) problems. LIVMARLI can cause stomach and intestinal problems, including diarrhea, stomach pain, and vomiting during treatment. Tell your healthcare provider right away if you have any of these symptoms more often or more severely than normal for you.
A condition called Fat Soluble Vitamin (FSV) Deficiency caused by low levels of certain vitamins (vitamin A, D, E, and K) stored in body fat. FSV deficiency is common in patients with Alagille syndrome but may worsen during treatment. Your healthcare provider should do blood tests before starting and during treatment.
Other common side effects reported during treatment were bone fractures and gastrointestinal bleeding.
About the Global Alagille Alliance (GALA)
Launched in October 2017, the Global ALagille Alliance (GALA) Study is advancing research and changing lives. By pooling together medical records from around the world to create a vast one-stop database of patient information, this three-year effort will be the bedrock of future research projects that will unravel the mysteries of Alagille Syndrome (ALGS). The cornerstone of this effort is a comprehensive medical record review of patients with ALGS up to 30 years of age from 1997 to present. GALA’s overarching aim is to conduct a comprehensive analysis of an international group of individuals with ALGS which will increase understanding of the disease and identify the challenges and needs of this unique population.
About Mirum Pharmaceuticals, Inc.
Mirum Pharmaceuticals, Inc. is a biopharmaceutical company dedicated to transforming the treatment of rare liver diseases. Mirum’s approved medication is LIVMARLI™ (maralixibat) oral solution which is approved in the U.S. for the treatment of cholestatic pruritus in patients with Alagille syndrome one year of age and older.
Mirum’s late-stage pipeline includes two investigational treatments for debilitating liver diseases affecting children and adults. Maralixibat (LIVMARLI), an oral ileal bile acid transporter (IBAT) inhibitor, is currently being evaluated in clinical trials for pediatric liver diseases and includes the MARCH Phase 3 study for progressive familial intrahepatic cholestasis (PFIC) and the EMBARK Phase 2b study for patients with biliary atresia. In addition, Mirum has an expanded access program open in Canada, Australia, the UK and several countries in Europe for eligible patients with Alagille syndrome.
Mirum has submitted a Marketing Authorization Application to the European Medicines Agency for LIVMARLI for the treatment of cholestatic liver disease in patients with Alagille syndrome.
Mirum’s second investigational treatment, volixibat, also an oral IBAT inhibitor, is being evaluated in two registrational studies including the OHANA Phase 2b study for pregnant women with intrahepatic cholestasis of pregnancy and the VISTAS Phase 2b study for adults with primary sclerosing cholangitis. Mirum is planning to launch a Phase 2b study in primary biliary cholangitis later this year.
To augment its pipeline in cholestatic liver disease, Mirum has acquired the exclusive option to develop and commercialize gene therapy programs VTX-803 and VTX-802 for PFIC3 and PFIC2, respectively, from Vivet Therapeutics SAS, following preclinical evaluation and investigational new drug-enabling studies.
Follow Mirum on Twitter, Facebook, LinkedIn and Instagram.
Forward-Looking Statements
This press release includes forward-looking statements pertaining to the Company’s planned participation at a scientific conference, which may include discussion of the Company’s product candidates and technologies, and the therapeutic potential thereof. Such forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements. Applicable risks and uncertainties include those relating to our preclinical research and clinical programs and other risks identified under the heading “Risk Factors” included in our most recent Form 10-Q and Form 10-K filings and in other future filings with the SEC. The forward-looking statements contained in this press release reflect Mirum's current views with respect to future events, and Mirum does not undertake and specifically disclaims any obligation to update any forward-looking statements.
The Liver Meeting® is a registered trademark of the American Association for the Study of Liver Diseases.
1Danks, et al. Archives of Disease in Childhood 1977
2Johns Hopkins Medicine. hopkinsmedicine.org/health/conditions-and-diseases/Alagille-syndrome
3Vandriel, et al. GALA EASL 2020; Kamath, et al. Hepatology Communications 2020
4Elisofon, et al. Journal of Pediatric Gastroenterology and Nutrition 2010
Contacts
Media:
Erin Murphy
media@mirumpharma.com
Investors:
Ian Clements, Ph.D.
ir@mirumpharma.com
Sam Martin
Argot Partners
ir@mirumpharma.com