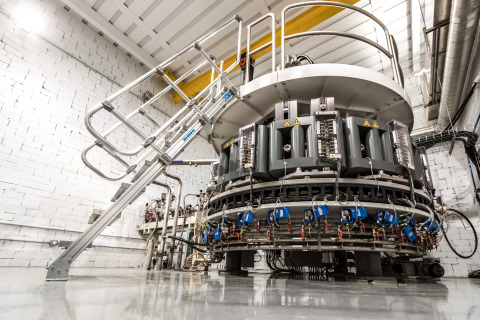
BELOIT, Wis.--(BUSINESS WIRE)--NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for medical imaging and therapeutic applications, hosted a growth and production expansion event, “Accelerating the Future of Patient Health,” at its headquarters in Beloit, Wis., on September 23, 2021. The event showcased the completion of NorthStar’s Accelerator Production and Isotope Processing facilities, which involved the installation of cutting-edge radioisotope production and processing equipment, and a groundbreaking celebration for its new state-of-the-art Therapeutic Radioisotope Production facility. Numerous guest speakers from national, state and local organizations joined with industry and healthcare leaders, NorthStar customers and investors to share perspectives on the exciting growth of nuclear medicine, the need for reliable U.S. radioisotope supply to support patient health and their experiences with NorthStar.
Guest speakers included:
- Keynote speaker Jeffrey Chamberlin, Associate Assistant Deputy Administrator for Material Management and Minimization, U.S. Department of Energy’s National Nuclear Security Administration, Washington, D.C.
- Diane M. Hendricks, Chairperson, Hendricks Holding Co., Inc.
- Lori S. Curtis Luther, City Manager, City of Beloit, Wis.
- Alan B. Packard, PhD, FSNMMI, Immediate Past President, Society of Nuclear Medicine and Molecular Imaging (SNMMI), Boston Children’s Hospital/Harvard Medical School, Boston, Mass.
- Randall C. Thompson, MD, FASNC, President, American Society of Nuclear Cardiology (ASNC), Professor of Medicine, University of Missouri-Kansas City School of Medicine, Saint Luke's Cardiovascular Consultants, Kansas City, Mo.
- Alan Taylor, PhD, Executive Chairman, Clarity Pharmaceuticals, Sydney, Australia
- Chris Vessell, U.S. Nuclear Medicine Supply Chain Leader, GE Healthcare, Arlington Heights, Ill.
- Tressa Snow, BS, CNMT, Nuclear Medicine Technologist, Beloit Health System
- Danny R. Allen, BCNP, President, NuTech Inc., Tyler, Texas
NorthStar Medical Radioisotopes is the sole commercial U.S. producer of the important medical radioisotope molybdenum-99 (Mo-99), and the only company in the world to use environmentally sound production processes that are non-uranium based. Mo-99 is used to generate technetium-99m (Tc-99m), which informs healthcare decisions for 40,000 U.S. patients daily. For nearly three years, NorthStar has provided the United States with reliable Mo-99 supply, used in its RadioGenix® System (technetium Tc 99m generator) to produce Tc-99m. NorthStar estimates that more than 1 million patients will have been diagnosed by the end of 2021 using NorthStar technology.
“NorthStar continues to make tremendous progress in our mission to provide patients global access to our game-changing radiopharmaceuticals,” said Stephen Merrick, President and Chief Executive Officer of NorthStar Medical Radioisotopes. “We are well on our way to having dual production and processing pathways for Mo-99 that give additional domestic production capacity and security of supply to better meet customer demand. Our two facility expansion projects in Beloit, Wis. are moving to the final phase of activities required for FDA and WI-DHS approval and will augment current Mo-99 production and processing in Columbia, Mo. The Isotope Processing facility in Beloit will enable us to more than double our current Mo-99 processing capabilities. The new Mo-99 Accelerator Production facility will add significant Mo-99 capacity and enable flexible scheduling, including Sunday production, which is highly desirable for our radiopharmacy customers. Testing of the Accelerator Production and Isotope Processing equipment is underway, with commercial production expected at the start of 2023, pending appropriate licensure and FDA approval.
“Importantly, NorthStar is rapidly expanding its leadership position in the emerging area of therapeutic radioisotopes, which are used in targeted radiopharmaceutical therapy to treat cancer, respiratory and other diseases. NorthStar is defining the supply chain for scalable, reliable therapeutic radioisotope production. By applying our proven Mo-99 production development expertise and environmentally sound electron accelerator technology, we are well-positioned to be the first commercial-scale producer of actinium-225 (Ac-225) and copper-67 (Cu-67). NorthStar is further investing in the future with our groundbreaking on a first-of-its-kind Therapeutic Radioisotope Production facility, exclusively dedicated to commercial-scale production of our no-carrier added (n.c.a.) Ac-225. In addition, we have expanded our pipeline with the recent addition of iodine-123 capsules to our future product offering, pending appropriate licensure and FDA approval, the development of our fibrin-specific diagnostic imaging candidate remains on track and we are evaluating additional potential opportunities with specialized SPECT radiopharmaceuticals and therapeutic radioisotopes to address unmet healthcare needs.”
Mr. Merrick continued, “We are extremely grateful to all of our partners and stakeholders in supporting our progress: the U.S. Department of Energy’s National Nuclear Security Administration and National Laboratories, the U.S. Food and Drug Administration, the Wisconsin Department of Health Services, our private commercial investors, NorthStar’s dedicated employees, our supply chain partners, and our customers. In particular, we would like to recognize the support that our partners at the University of Missouri Research Reactor (MURR®), with whom we jointly produce Mo-99 in Columbia, Missouri, continue to provide to NorthStar. IBA (Ion Beam Applications S.A., EURONEXT), a leading global supplier of accelerators that is focused on bringing integrated and innovative solutions for the diagnosis and treatment of cancer, continues to be a tremendous collaborator in our efforts. We are also proud to help showcase both the City of Beloit and Wisconsin as pioneers and leaders in technology innovation and sustainable radioisotope production. Our expansion activities are supported by Corporate Contractors Incorporated (CCI), the lead contractor, Springs ATG (Advanced Technology Group) and Von Gahlen, a leading global supplier of state-of-the-art shielding solutions for nuclear medicine and radiopharmacy.”
“This is a very exciting time for NorthStar and everyone in the nuclear medicine community. All of us share a vision to accelerate the future of patient health by providing innovative solutions to ensure reliable access to radioisotopes that can make a positive difference in healthcare for people around the world,” he concluded.
About NorthStar Medical Radioisotopes, LLC (NorthStar)
NorthStar Medical Radioisotopes is a commercial-stage nuclear medicine company that develops, produces and manufactures reliable and environmentally-friendly diagnostic and therapeutic radiopharmaceuticals. Its first FDA-approved diagnostic imaging product is technetium-99m (Tc-99m), which is used in 40,000 patient imaging studies per day in the United States as standard of care to assess extent and severity of heart disease and cancer. Tc-99m is generated by NorthStar’s novel RadioGenix® System (technetium Tc 99m generator) which uses U.S.-produced, non-uranium based molybdenum-99 (Mo-99) as its source material. The Company is executing a well-defined plan to consistently increase the scale of Mo-99 production and to continuously improve efficiencies to meet anticipated increased demand. Therapeutic radioisotopes are increasingly important cancer treatment options, and NorthStar is developing commercial-scale production technologies to meet high demand for their use in ongoing clinical trials by multiple pharmaceutical companies. In addition, the Company is advancing a portfolio of other radiopharmaceuticals for use in therapeutic and diagnostic applications. For more information, visit: www.northstarnm.com.