PARIS--(BUSINESS WIRE)--Regulatory News:
GenSight Biologics (Paris:SIGHT) (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today announced that Eye, the official journal of the Royal College of Ophthalmologists (UK), has published the final results of the REALITY Leber Hereditary Optic Neuropathy (LHON) Registry study. The paper*, published in the April 28 issue of Eye under the title “Natural History of Patients with Leber Hereditary Optic Neuropathy – Results from the REALITY study”, was a retrospective study of 44 LHON patients whose data were collected from a period spanning the pre-symptomatic stage of the disease to at least more than one year after onset of vision loss (chronic stage).
The investigators analyzed the natural history visual outcomes of patients with Leber Hereditary Optic Neuropathy (LHON) who carried one of the three primary mitochondrial DNA (mtDNA) mutations that cause approximately 90% of all cases. One of the main findings is that the worst outcomes were reported in patients with LHON caused by the m.11778G>A mutation in the ND4 gene, who were aged at least 15 years old at onset. Statistical modeling of the evolution of best-corrected visual acuity (BCVA) over time found no tendency for spontaneous recovery, instead depicting a severe and permanent deterioration of BCVA.
“The REALITY study confirms the devastating effect of the m.11778G>A mutation on vision and the poor visual prognosis, more so for patients who are 15 years or older at the time of disease onset,” commented lead author Dr. Patrick Yu-Wai-Man, MD, PhD, REALITY principal investigator and Associate Professor and Honorary Consultant Ophthalmologist at the University of Cambridge, Moorfields Eye Hospital, and the UCL Institute of Ophthalmology, London, United Kingdom.
REALITY and LHON Natural History
REALITY was an international observational retrospective registry of LHON patients naïve to gene therapy conducted in 11 centers in France, Italy, Spain, the UK and the US. Of the 44 patients who were enrolled, a subset of 23 carried the m.11778G>A mutation in the ND4 gene and were at least 15 years old at onset of vision loss. Age and gender distribution in this subset of patients matched those of the subjects treated with LUMEVOQ® in the RESCUE and REVERSE clinical trials.
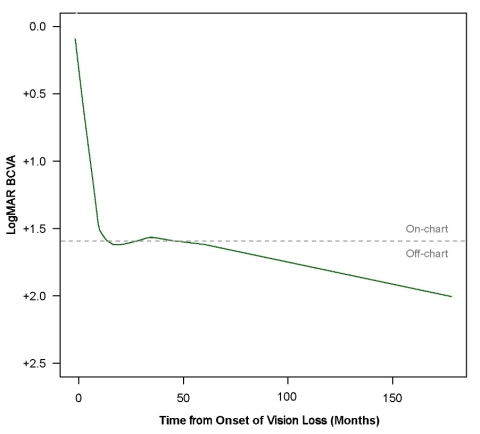
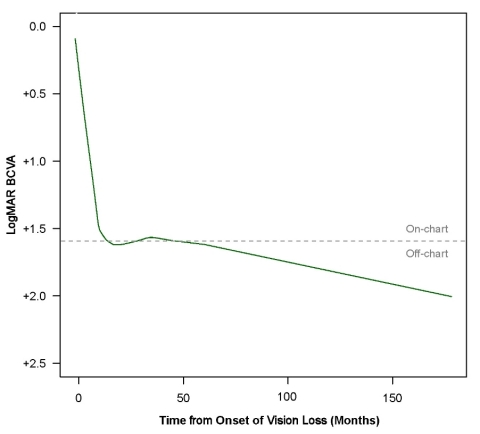
At the last observation available (on average 3 years after onset of symptoms), these patients had a mean BCVA of 1.55 LogMAR, equivalent to a Snellen fraction of 20/700. Locally Weighed Scatterplot Smoothing (LOWESS) regression analysis was used to model the evolution of BCVA in the ND4 LHON patients aged at least 15 years old. The fitted curve showed an initial marked loss of vision during the acute phase of the disease, followed by a continuous deterioration of BCVA over the 3-year follow-up, with no trend for spontaneous visual recovery. Notably, half of the eyes in these patients had off-chart visual acuity at the last available observation.
LogMAR: logarithm of the minimal angle resolution.
The curve depicts the Locally Weighed Scatterplot Smoothing (LOWESS) regression analysis of 213 individual BCVA data points collected in 23 LHON patients with the ND4 mutation who were ≥ 15 at onset.
These findings for LHON patients with the m.11778G>A mutation are consistent with a recent meta-analysis of the literature, which reported a severe visual loss with rare or poor spontaneous recovery in this population.a
The pattern of a sharp deterioration of visual acuity followed by an extended period of low acuity stands in stark contrast against the improvements observed in the RESCUE and REVERSE trials.
The paper is available at https://doi.org/10.1038/s41433-021-01535-9.
*About the paper:
Natural History of Patients with Leber Hereditary Optic Neuropathy – Results from the REALITY Study
Authors: Patrick Yu-Wai-Man,1,2,3,4 Nancy J Newman,5 Valerio Carelli,6,7 Chiara La Morgia,6,7 Valérie Biousse,5 Francesco M Bandello,8,9 Catherine Vignal Clermont,10,11 Lorena Castillo Campillo,12 Stephanie Leruez,13 Mark L Moster,14 Dean M Cestari,15 Rod Foroozan,16 Alfredo Sadun,17 Rustum Karanjia, 17,18 Neringa Jurkute,3,4 Laure Blouin,19 Magali Taiel,19 José-Alain Sahel,20,21,22,23 for the LHON REALITY Study Group.
Affiliations:
1Cambridge Centre for Brain Repair and MRC Mitochondrial Biology Unit, Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK.
2Cambridge Eye Unit, Addenbrooke’s Hospital, Cambridge University Hospitals, Cambridge, UK.
3Moorfields Eye Hospital, London, UK.
4UCL Institute of Ophthalmology, University College London, London, UK.
5Departments of Ophthalmology, Neurology and Neurological Surgery, Emory University School of Medicine, Atlanta, Georgia USA.
6IRCCS Istituto delle Scienze Neurologiche di Bologna, UOC Clinica Neurologica, Bologna, Italy
7Unit of Neurology, Department of Biomedical and Neuromotor Sciences (DIBINEM), University of Bologna, Bologna, Italy
8Department of Ophthalmology Vita-Salute San Raffaele University, Milan, Italy
9IRCCS San Raffaele Scientific Institute, Milan, Italy
10Department of Neuro Ophthalmology and Emergencies, Rothschild Foundation Hospital, Paris, France
11Centre Hospitalier National d’Ophtalmologie des Quinze Vingts, Paris, France
12Institut Catala de Retina, Barcelona, Spain.
13CHU Angers - Hôpital Hôtel Dieu, Angers, France.
14Departments of Neurology and Ophthalmology, Wills Eye Hospital and Thomas Jefferson University, Philadelphia, PA, USA
15Massachusetts Eye and Ear Infirmary, Boston, MA, USA.
16Alkek Eye Center, Houston, TX, USA.
17Doheny Eye Center UCLA, Department of Ophthalmology David Geffen School of Medicine at UCLA, Doheny Eye Institute, Los Angeles, CA, USA
18Department of Ophthalmology, University of Ottawa Eye, Ottawa ON Canada
19GenSight Biologics, Paris, France
20Sorbonne Université, INSERM, CNRS, Institut de la Vision, 75012 Paris, France
21Fondation Ophtalmologique A. de Rothschild, 25-29 rue Manin, 75019 Paris
22Department of Ophthalmology, The University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
23CHNO des Quinze-Vingts, Institut Hospitalo-Universitaire FOReSIGHT, INSERM-DGOS CIC 1423, Paris, France
Notes:
a Newman NJ, Carelli V, Taiel M, Yu-Wai-Man P. Visual Outcomes in Leber Hereditary Optic Neuropathy Patients with the m.11778G>A (MTND4) Mitochondrial DNA Mutation. Journal of Neuro-ophthalmology. 2020 Dec;40(4):547-557.
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics’ lead product candidate, LUMEVOQ® (GS010; lenadogene nolparvovec), has been submitted for marketing approval in Europe for the treatment of Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease affecting primarily teens and young adults that leads to irreversible blindness. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About Leber Hereditary Optic Neuropathy (LHON)
Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1st eye, with the 2nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of patients have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously. The estimated incidence of LHON is approximately 800-1,200 new patients who lose their sight every year in the United States and the European Union.
About LUMEVOQ® (GS010; lenadogene nolparvovec)
LUMEVOQ® (GS010; lenadogene nolparvovec) targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function. “LUMEVOQ” was accepted as the invented name for GS010 (lenadogene nolparvovec) by the European Medicines Agency (EMA) in October 2018.
About RESCUE and REVERSE
RESCUE and REVERSE are two separate randomized, double-masked, sham-controlled Phase III trials designed to evaluate the efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in subjects affected by LHON due to the G11778A mutation in the mitochondrial ND4 gene.
The primary endpoint measured the difference in efficacy of GS010 in treated eyes compared to sham-treated eyes based on Best-Corrected Visual Acuity (BCVA), as measured with the ETDRS at 48 weeks post-injection. The patients’ LogMAR (Logarithm of the Minimal Angle of Resolution) scores, which are derived from the number of letters patients read on the ETDRS chart, was used for statistical purposes. Both trials were adequately powered to evaluate a clinically relevant difference of at least 15 ETDRS letters between treated and untreated eyes adjusted to baseline.
The secondary endpoints involved the application of the primary analysis to best-seeing eyes that received GS010 compared to those receiving sham, and to worse-seeing eyes that received GS010 compared to those that received sham. Additionally, a categorical evaluation with a responder analysis was evaluated, including the proportion of patients who maintain vision (< ETDRS 15L loss), the proportion of patients who gain 15 ETDRS letters from baseline and the proportion of patients with Snellen acuity of >20/200. Complementary vision metrics included automated visual fields, optical coherence tomography, and color and contrast sensitivity, in addition to quality of life scales, bio-dissemination and the time course of immune response. Readouts for these endpoints were at 48, 72 and 96 weeks after injection.
The trials were conducted in parallel, in 37 subjects for REVERSE and 39 subjects for RESCUE, in 7 centers across the United States, the UK, France, Germany and Italy. Week 96 results were reported in 2019 for both trials, after which patients were invited to a long-term follow-up study that will last for three years.
ClinicalTrials.gov Identifiers:
REVERSE: NCT02652780
RESCUE: NCT02652767
About REALITY
REALITY is a multi-country retrospective and cross-sectional observational study of affected LHON subjects, based on subjects’ medical charts and the administration of surveys on Health-Related Quality of Life (HRQoL) and direct and indirect costs associated with the disease. The study enrolled 44 subjects (both adult and pediatric) chiefly in the following countries: Spain, Italy, France, United Kingdom, and the United States.
The primary objective for the REALITY study was to describe the evolution of visual functional and structural changes and other associated symptoms in patients with LHON; understand the impact of LHON-related vision loss on the HRQoL; and understand the economic burden for patients and their families arising from direct and indirect costs associated with the disease. The secondary objective is to describe the relationship between genetic, lifestyle and/or environmental factors and the expression of the LHON phenotype.
The first subject was enrolled on 3 January 2018, and enrollment was completed in early Q2 2020.
ClinicalTrials.gov Identifiers:
REALITY LHON Registry: NCT03295071