COLUMBIA, Md.--(BUSINESS WIRE)--Ambu Inc., a rapidly growing medical device maker and pioneer of sterile, single-use endoscopes, announced today that it has received Health Canada clearance for the aScope™ 4 Cysto, the company’s innovative flexible cystoscope platform for urology. The launch will give urologists across Canada immediate access to a single-use cystoscope option for diagnosing, managing, and treating lower urinary disorders such as hematuria, incontinence, and bladder cancer.
“We are excited to bring our single-use cystoscope to urologists in Canada,” said Steven Block, President of Ambu Inc. “Since launching in the United States, we have seen the positive impact our technology can have – redefining productivity for the doctors, staff, and the facility, while ensuring a safe, sterile device for the patient.”
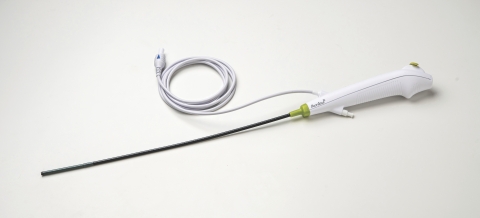
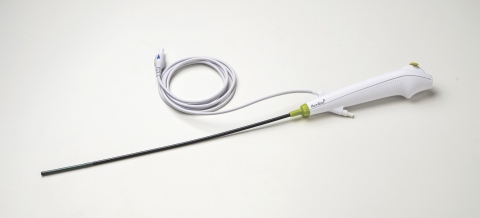
Ambu’s aScope 4 Cysto is a cost-effective, high-quality flexible endoscope featuring excellent maneuverability and optics. Unlike traditional reusable endoscopes, single-use eliminates the significant capital, repair, and cleaning costs required to ensure a patient-ready instrument for every procedure.
Each aScope 4 cystoscope is sterile from the package and ready when needed, enabling facilities to improve workflow efficiencies and reallocate staff from complex, time-consuming endoscope cleaning to more productive activities.
“We know there are challenges in today’s urology clinics – such as COVID-19-created procedure backlogs – that the aScope 4 Cysto can directly address,” said Doug Pedersen, Ambu Canada’s National Sales Director. “By providing a new paradigm for increasing outpatient throughput that isn’t constrained by reprocessing or capital budgets, we can improve urology clinic workflows and patient scheduling flexibility.”
Ambu’s new cystoscope is utilized with the company’s aView™ 2 Advance, a state-of-the-art, high-definition display for video endoscopy. The compact and portable unit is easily transported throughout the hospital or from room to room, can be connected to EHR/EMR systems, and provides image and video procedure recording for documentation or for immediate review with the patient. The aView 2 Advance platform supports the company’s entire line of single-use flexible endoscopes for cystoscopy, bronchoscopy, and rhinolaryngoscopy.
20 new endoscopy devices over the next three years
Ambu launched the world’s first single-use flexible bronchoscope, the Ambu® aScope™ in 2009. In 2020, more than 1 million Ambu single-use endoscopes were used in more than 6,000 hospitals making Ambu the world’s largest supplier of single-use endoscopes.
Ambu launched three new products in its Visualization business in 2020. The company received 510(k) clearance from the U.S. Food and Drug Administration (FDA) in April 2020 for its single-use cystoscope, and in July 2020 the FDA cleared Ambu’s single-use duodenoscope. Ambu introduced the aView 2 Advance in May 2020. By 2023, Ambu expects to introduce another 20 new devices across all major areas of endoscopy, including GI, the largest endoscopy market globally.
About Ambu
Ambu has been bringing the solutions of the future to life since 1937. Today, millions of patients and healthcare professionals worldwide depend on the efficiency, safety and performance of our single-use endoscopy, anaesthesia, and patient monitoring & diagnostics solutions. The manifestations of our efforts have ranged from early innovations like the Ambu® Bag™ resuscitator and the Ambu® BlueSensor™ electrodes to our newest landmark solutions like the Ambu® aScope™ – the world’s first single-use flexible endoscope. Moreover, we continuously look to the future with a commitment to deliver innovative quality products that have a positive impact on the work of doctors, nurses and paramedics. Headquartered near Copenhagen in Denmark, Ambu employs approximately 4,000 people in Europe, North America and the Asia Pacific. For more information, please visit ambu.com or ambuUSA.com.