AUSTIN, Texas--(BUSINESS WIRE)--Maxwell Biosciences, a preclinical stage biotechnology company developing CLAROMER™ brand anti-infectives, today announced that the peer-reviewed, open-access journal Pharmaceuticals (MDPI) has published new scientific research findings co-authored by Scientific Advisory Board Member Gill Diamond, Ph.D. (University of Louisville, Department of Oral Microbiology and Infectious Diseases) and eleven other collaborating academic researchers. Titled Potent Antiviral Activity against HSV-1 and SARS-CoV-2 by Antimicrobial Peptoids, the research findings demonstrate that several peptoids exhibit potent in vitro antiviral activity against both HSV-1 and SARS-CoV-2.
As outlined in the paper’s abstract, viral infections, such as those caused by Herpes Simplex Virus-1 (HSV-1) and SARS-CoV-2, affect millions of people each year. However, there are few drugs that treat viral infections effectively, and no vaccine to prevent HSV-1 infections exists.
“There’s a huge need for new antiviral agents,” said Dr. Diamond, “and whereas new vaccines are now available for SARS-CoV-2, treatments can help those who become infected and develop Covid-19 illness. This paper constitutes the first report of biomimetic antiviral peptoids—stable mimics of natural antiviral peptides—that effectively inactivate two different enveloped viruses, utilizing a mechanism of action similar to that of natural innate immunity. Recurrent HSV-1 infections affect around 177M adult Americans; and of course, SARS-CoV-2 affects us all.”
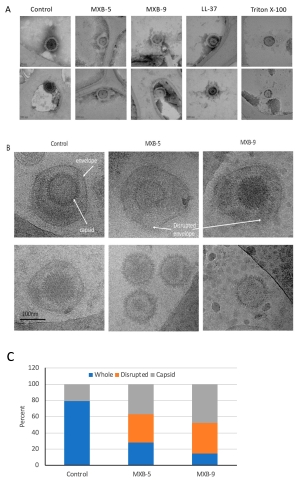
As detailed in the body of the paper and visualized by cryo-EM images the researchers showed experimentally that antiviral peptoids disrupt the phospholipid envelopes of the viruses by a mechanism similar to that observed for natural human antiviral peptides.
Dr. Diamond stated, “The entire research team involved in this study deserves tremendous praise, as interdisciplinary research of this nature is critical to the development of new therapies that are effective against viral infections, while also being safe for human use.” Dr. Diamond is continuing to study these promising peptoids now as topical treatments in a rodent model of Herpes Labialis.
Key Observations and Findings
- The antiviral peptoids were synthesized at the DOE-sponsored Molecular Foundry. Their antiviral activity was studied and tested at the University of Louisville with financial support from Maxwell Biosciences. They were designed to function as mimics of natural biological antiviral peptides. Antiviral peptoids have the distinct advantage of being insensitive to the proteases that quickly degrade biological peptides, and thus offer increased bioavailability and stability in the body.
- Herpes simplex virus type-1 (HSV-1) infections cause recurrent oral lesions and affect millions in the developed world. HSV-1 is also a major cause of infectious blindness and genital infections worldwide. HSV-1 infections are life-threatening in immunocompromised individuals [1]. Furthermore, there is recent evidence that HSV-1 infections are associated with the pathogenesis of Alzheimer’s disease, magnifying the importance of treating these infections early [2]. HSV-1 virus is transmitted readily through oral secretions. It is estimated that up to 80% of the population is infected with this virus, depending on age and socioeconomic status [3].
- The innate immune system is one of the primary mechanisms for recognizing and eliminating viruses and other pathogens from mucosal surfaces (reviewed in [6]).
- Challenges of using natural antimicrobial peptides as therapeutics spurred the development of non-natural peptidomimetics such as ‘peptoids’, which are a class of biostable, sequence-specific N-substituted glycine oligomers [14,15].
- Based on the prior results demonstrating the inactivation of enveloped viruses by antiviral peptides via membrane-disruption mechanisms, Drs. Diamond and Barron hypothesized that antimicrobial peptoids designed to mimic natural antiviral peptides could inactivate an enveloped virus such as HSV-1. And since the recently emerged virus SARS-CoV-2 (the etiological agent of COVID-19) is similarly enveloped, they further believed that these peptoids might exhibit activity against this devastating virus.
- A library of ten different biomimetic peptoids was tested, by comparison to the natural human host defense peptide LL-37 (the only human form of cathelicidin antimicrobial peptides) for activity against HSV-1. LL-37 is a broad-spectrum antiviral peptide.
- The researchers’ previous results demonstrated that the natural human antimicrobial peptide LL-37 disrupts the membrane of Kaposi’s Sarcoma Herpes Virus (KSHV) [8]. Thus, they hypothesized that these peptoids, which are simplified structural mimics of natural antimicrobial peptides, could act through a similar biophysical mechanism.
- To determine whether the peptoids exhibited activity against enveloped viruses, the researchers tested the in vitro inhibitory activity of peptoids MXB-4 and MXB-9 against SARS-CoV-2. When incubated with virus for 1 h at 37 °C at increasing concentrations, they observed virus inactivation with IC50 values of 20 µg/mL and 7 µg/mL, respectively (Figure 4). These two different peptoids were also well tolerated by human cells.
- To determine whether the peptoids acted against SARS-CoV-2 by a membrane-dependent mechanism as with HSV-1, the researchers treated SARS-CoV-2 with the active antiviral peptoids (MXB-4 and MXB-9), followed by visualization by Cryo-EM, and confirmed that this was the case; direct disruption of the viral membrane was observed.
- To provide further evidence to support the development of antiviral peptoids as potential therapeutics, Dr. Diamond et al. assayed peptoid cytotoxicity against cultured human cells, and observed excellent tolerability up to 500 µg/mL. Thus, these antiviral peptoids offer an apparently excellent therapeutic window.
For Discussion
- Herpesvirus infections are common and easily transmissible. However, due to the mechanisms of infection and intraneuronal pathogenesis utilized by HSV-1, it has been difficult to design vaccines that can prevent herpesvirus infections. Furthermore, other preventive and palliative methods have been challenging to adapt to broad clinical use. Since antimicrobial peptides such as LL-37 exhibit potent activity against HSV-1 [36], their potential as antiviral preventive or therapeutic agents is significant. However, direct introduction of these peptides has not translated into effective treatments, due to high cost, molecular instability, and unknown pharmacokinetics, among other problems [37,38]. Here, the researchers have demonstrated the potential use of novel, biostable AMP-mimetic compounds which, based on their mechanism of action, not only inactivate HSV-1, but also can be used against other, potentially more dangerous enveloped viral pathogens such as SARS-CoV-2.
- Lack of cytotoxicity for the host cell is important for any therapeutic. Since HSV-1 generally affects oral epithelial tissues, the researchers tested the active peptoids on well-differentiated 3D cultures of oral epithelium. The drugs were applied to the apical surface, and showed no cytotoxic effects after a 3-h exposure. This is in line with results routinely obtained with AMPs such as LL-37, which only exhibits cytotoxic effects on host cells at high concentrations [40].
- The new results strongly suggest that, like known AMP activity against bacteria and fungi, the peptoids target and disrupt pathogenic membranes, while being safe for host cells.
- Regardless of the specific molecular mechanism, the results suggest that peptoids of the type described herein could be developed as broad-spectrum antivirals that can target numerous enveloped viruses, including those which are highly pathogenic.
Links
- Research Abstract as Published in MDI
- Full Study as Published in MDI
- Figures and Images as Published in MDI
The MDI Article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
About Maxwell Biosciences
Founded in 2016, Maxwell Biosciences develops drugs designed to be effective against rapidly evolving, widespread viruses. Maxwell’s CLAROMER™ brand anti-infectives destroy a broad spectrum of viruses, as shown in preclinical studies including imaging of viral structures. The drugs were shown in ongoing preclinical studies to inactivate multiple Coronavirus strains, Herpes Simplex Virus 1, Hepatitis B and C, and Influenza viruses. They have also been shown to be well tolerated in human tissues and in vivo animal studies. FDA IND-enabling studies are ongoing. Maxwell’s technology is protected by six granted patents and is led by a world-class team of experienced life sciences executives. To learn more, visit www.maxwellbiosciences.com.